
What is known as the triple point of water?
A) When the boiling point and freezing point of water become the same.
B) When the boiling point and freezing point of water become different.
C) When the boiling point and freezing point of water become the opposite.
D) None of the above.
Answer
578.4k+ views
Hint:There are basically three phases in which a substance can exist – solid, liquid and gas. Triple point corresponds to conditions at which the three phases of a substance can coexist. For water, the liquid form is water, its solid form refers to ice and its gaseous form is steam.
Complete step by step answer.
Step 1: Based on the definition of the triple point of a substance determine the conditions to be satisfied for the triple point of water.
We know the three phases of water are water, steam and ice.
Lowering the temperature causes steam to condense and become water and if the temperature is again lowered it solidifies to form ice.
The temperature at which water boils is $100^\circ {\text{C}}$ and the temperature at which it freezes is $0^\circ {\text{C}}$ .
At the triple point, all three phases of water must coexist i.e., ice, water and steam have to coexist. For this, the boiling point and freezing point of water has to be the same.
The graph given below depicts the triple point of water.
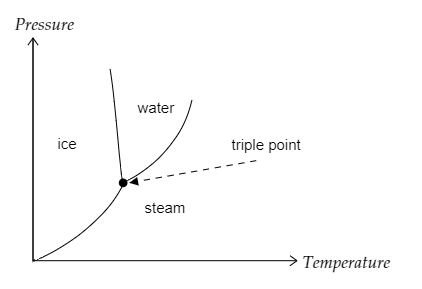
So the correct option is A.
Note:Water can boil and freeze at other temperatures as well. The pressure is the factor which helps to achieve the triple point. The boiling point can be decreased by decreasing the pressure. Simultaneously the freezing point of water will increase. It is only at one specific pressure and temperature at which water, ice and steam coexist and it is found to be at temperature $0 \cdot 01^\circ {\text{C}}$ and pressure $611 \cdot 2{\text{Pa}}$ .
Complete step by step answer.
Step 1: Based on the definition of the triple point of a substance determine the conditions to be satisfied for the triple point of water.
We know the three phases of water are water, steam and ice.
Lowering the temperature causes steam to condense and become water and if the temperature is again lowered it solidifies to form ice.
The temperature at which water boils is $100^\circ {\text{C}}$ and the temperature at which it freezes is $0^\circ {\text{C}}$ .
At the triple point, all three phases of water must coexist i.e., ice, water and steam have to coexist. For this, the boiling point and freezing point of water has to be the same.
The graph given below depicts the triple point of water.

So the correct option is A.
Note:Water can boil and freeze at other temperatures as well. The pressure is the factor which helps to achieve the triple point. The boiling point can be decreased by decreasing the pressure. Simultaneously the freezing point of water will increase. It is only at one specific pressure and temperature at which water, ice and steam coexist and it is found to be at temperature $0 \cdot 01^\circ {\text{C}}$ and pressure $611 \cdot 2{\text{Pa}}$ .
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




