
KI in acetone, undergoes ${{S}_{N}}2$ reaction with each of P, Q, R and S. the rates of the reaction vary as _____.
P > Q > R > S
S > P > R > Q
Q > R > P> S
R > P > S > Q
Answer
559.5k+ views
Hint: ${{S}_{N}}2$ Reaction are nucleophilic substitution reaction, it involves the attack of a positively charged or a partially positively charged atom or group by a nucleophile. Nucleophiles are the species which are rich in electrons; they can donate an electron pair.
Complete step by step answer:
- ${{S}_{N}}2$ Reaction follows the second order kinetics and it is a single step reaction. The rate of the reaction depends upon the concentration of the substrate and nucleophile. There is a formation of a single transition state in ${{S}_{N}}2$ reaction.
- ${{S}_{N}}2$ Reaction leads to a back-side attack, which leads to the inversion of stereochemistry of the carbon atom, here a complete inversion of configuration takes place.
Mechanism of ${{S}_{N}}2$ reaction is mentioned below:
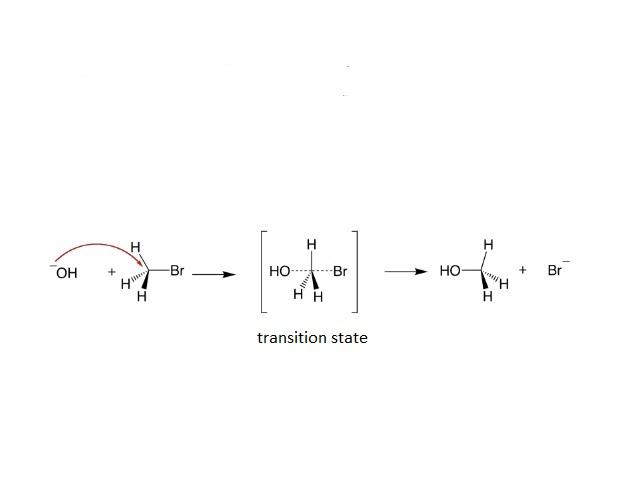
- Methyl halides are more reactive than the primary alkyl halides towards the ${{S}_{N}}2$ reaction. Similarly the primary alkyl halides are more reactive towards the ${{S}_{N}}2$ reaction then the secondary halides.
- This is due to the steric hindrance and the electron donating effect of the alkyl group which increases the electron density on the carbon which is attached to the halogen and makes it less electrophilic.
Note: For ${{S}_{N}}2$ reaction higher will be the temperature more elimination product we get. The more elimination products we get, the substitution product will be less because the amount of the reactant is limited. This is because the activation energy for a particular reaction is higher for elimination reaction than the substitution reaction for the same reaction.
Complete step by step answer:
- ${{S}_{N}}2$ Reaction follows the second order kinetics and it is a single step reaction. The rate of the reaction depends upon the concentration of the substrate and nucleophile. There is a formation of a single transition state in ${{S}_{N}}2$ reaction.
- ${{S}_{N}}2$ Reaction leads to a back-side attack, which leads to the inversion of stereochemistry of the carbon atom, here a complete inversion of configuration takes place.
Mechanism of ${{S}_{N}}2$ reaction is mentioned below:

- Methyl halides are more reactive than the primary alkyl halides towards the ${{S}_{N}}2$ reaction. Similarly the primary alkyl halides are more reactive towards the ${{S}_{N}}2$ reaction then the secondary halides.
- This is due to the steric hindrance and the electron donating effect of the alkyl group which increases the electron density on the carbon which is attached to the halogen and makes it less electrophilic.
Note: For ${{S}_{N}}2$ reaction higher will be the temperature more elimination product we get. The more elimination products we get, the substitution product will be less because the amount of the reactant is limited. This is because the activation energy for a particular reaction is higher for elimination reaction than the substitution reaction for the same reaction.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




