
${K_2}C{r_2}{O_7}$ when reacts with cold conc. ${H_2}S{O_4}$ gives red crystal of
A $Cr{O_4}^{2 - }$
B $Cr{O_3}$
C $C{r_2}{(S{O_4})_3}$
D $C{r_2}{O_3}$
Answer
571.8k+ views
Hint: Potassium dichromate that is ${K_2}C{r_2}{O_7}$is a good oxidising agent and also a good dehydrating agent. When it reacts with cold conc. ${H_2}S{O_4}$ it gives a red crystal of chromic(VI) oxide.
Complete step by step answer:
Chromic(VI) oxide that is written as $Cr{O_3}$can be prepared when concentrated sulphuric is added to a saturated solution of potassium dichromate.
Chromic(VI) oxide crystals are red in colour, here in $Cr{O_3}$ the oxidation number of chromium ions is $ + 6$.
The balanced chemical equation can be written as when potassium dichromate reacts with cold sulphuric acid is:
${K_2}C{r_2}{O_7} + \mathop {{H_2}S{O_4}}\limits_{Conc.} \to {K_2}S{O_4} + 2Cr{O_3} + {H_2}O$
Here the chromium oxide $Cr{O_3}$ formed is an acidic oxide, therefore it readily reacts with alkalis. It reacts with sodium hydroxide to give sodium chromate and water.The balanced chemical reaction is given as:
$Cr{O_3} + NaOH \to N{a_2}Cr{O_4} + {H_2}O$
Chromium oxide $Cr{O_3}$ also dissolves in water to yield chromic acid that is ${H_2}Cr{O_4}$. The balanced chemical equation is given as:
$Cr{O_3} + {H_2}O \to {H_2}Cr{O_4}$
So, the correct answer is Option B.
Note: Potassium dichromate that is ${K_2}C{r_2}{O_7}$is an orange solid and it is not soluble in water. It is an analytical reagent and is preferred over sodium dichromate since the latter is hygroscopic in nature. The structure of potassium dichromate consists of two tetrahedral chromate units which are linked together by an oxygen atom.
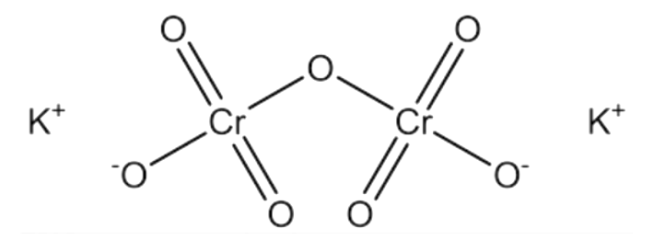
It should be noted that chromium shows an array of oxidation state from II to VI. The highest oxidation state VI however only exists in oxo species like chromium(VI) oxide $Cr{O_3}$.
Complete step by step answer:
Chromic(VI) oxide that is written as $Cr{O_3}$can be prepared when concentrated sulphuric is added to a saturated solution of potassium dichromate.
Chromic(VI) oxide crystals are red in colour, here in $Cr{O_3}$ the oxidation number of chromium ions is $ + 6$.
The balanced chemical equation can be written as when potassium dichromate reacts with cold sulphuric acid is:
${K_2}C{r_2}{O_7} + \mathop {{H_2}S{O_4}}\limits_{Conc.} \to {K_2}S{O_4} + 2Cr{O_3} + {H_2}O$
Here the chromium oxide $Cr{O_3}$ formed is an acidic oxide, therefore it readily reacts with alkalis. It reacts with sodium hydroxide to give sodium chromate and water.The balanced chemical reaction is given as:
$Cr{O_3} + NaOH \to N{a_2}Cr{O_4} + {H_2}O$
Chromium oxide $Cr{O_3}$ also dissolves in water to yield chromic acid that is ${H_2}Cr{O_4}$. The balanced chemical equation is given as:
$Cr{O_3} + {H_2}O \to {H_2}Cr{O_4}$
So, the correct answer is Option B.
Note: Potassium dichromate that is ${K_2}C{r_2}{O_7}$is an orange solid and it is not soluble in water. It is an analytical reagent and is preferred over sodium dichromate since the latter is hygroscopic in nature. The structure of potassium dichromate consists of two tetrahedral chromate units which are linked together by an oxygen atom.

It should be noted that chromium shows an array of oxidation state from II to VI. The highest oxidation state VI however only exists in oxo species like chromium(VI) oxide $Cr{O_3}$.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




