
What is the IUPAC name of the compound?
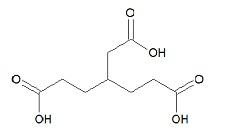

Answer
522.9k+ views
Hint: Identification of functional groups, number of carbon atoms
Presence of any branch group and its corresponding position help you to find the name of the compound.
Complete answer:
Identification of function group R-COOH, which is a carboxylic acid
Number of carbon atom is $6$
Number of hydrogen atom is $8$
Number of oxygen atom is $6$
Therefore, ${{C}_{6}}{{H}_{8}}{{O}_{6}}$
Hence the name of the group can be said as propane or (${{C}_{3}}{{H}_{8}}$) or tri
It contains three carboxyl groups
The position of carboxyl branch can be mentioned as $1,2,3$
Combining all these we can predict the name of the compound and its molecular formula
Hence the name of the compound is identified as Tri-Carballylic acid
It can also be called as Propane- $1,2,3-$Carboxylic acid
It can be also referred to as carballylic acid and β-carboxyglutaric acid
It is formed by the conjugate of tricarballylate
It can act as an inhibitor for the enzymes
It interferes with Krebs cycle
It can be synthesized from Fumaric acid
Tri-Carballylic acid looks similar to citric acid which lacks the hydroxide group
It belongs to the group of carboxylic acid
Tricarboxylic acid involves in the cellular respiration, breakdown of cells thereby helps in the growth and division of cells
Note:
You can directly identify the IUPAC name by calculating the number of C, H, O and their corresponding functional groups
It is necessary to know the similar names or similar structure compounds of carboxylic acid such as Propane- 1,2,3 -Carboxylic acid, Carballylic acid and β-carboxyglutaric acid
Presence of any branch group and its corresponding position help you to find the name of the compound.
Complete answer:
Identification of function group R-COOH, which is a carboxylic acid
Number of carbon atom is $6$
Number of hydrogen atom is $8$
Number of oxygen atom is $6$
Therefore, ${{C}_{6}}{{H}_{8}}{{O}_{6}}$
Hence the name of the group can be said as propane or (${{C}_{3}}{{H}_{8}}$) or tri
It contains three carboxyl groups
The position of carboxyl branch can be mentioned as $1,2,3$
Combining all these we can predict the name of the compound and its molecular formula
Hence the name of the compound is identified as Tri-Carballylic acid
It can also be called as Propane- $1,2,3-$Carboxylic acid
It can be also referred to as carballylic acid and β-carboxyglutaric acid
It is formed by the conjugate of tricarballylate
It can act as an inhibitor for the enzymes
It interferes with Krebs cycle
It can be synthesized from Fumaric acid
Tri-Carballylic acid looks similar to citric acid which lacks the hydroxide group
It belongs to the group of carboxylic acid
Tricarboxylic acid involves in the cellular respiration, breakdown of cells thereby helps in the growth and division of cells
Note:
You can directly identify the IUPAC name by calculating the number of C, H, O and their corresponding functional groups
It is necessary to know the similar names or similar structure compounds of carboxylic acid such as Propane- 1,2,3 -Carboxylic acid, Carballylic acid and β-carboxyglutaric acid
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

What organs are located on the left side of your body class 11 biology CBSE

Draw a diagram of nephron and explain its structur class 11 biology CBSE




