
What is the IUPAC name of acetylation product of \[anisole\]?
Answer
502.2k+ views
Hint: First step to solve the answer is to find the acetylation product of \[anisole\]. Then following the IUPAC rule name the product formed by this process. In acetylation an acetyl group is added to the aromatic ring.
Complete Answer:
The process where an acetyl group is added to a compound because of its replacement with that of an active \[hydrogen\] atom is called acetylation. An acetyl group is included in the organic compound, such a compound is termed as acetates or acetate esters. Acetyl group consists of carbonyl atoms or a \[carbon\] atom bonded in double pairs with \[oxygen\] or a methyl group on end.
Now that we know the acetylation process, we can determine the product.
When \[anisole\]reacts with acetyl chloride it yields a ketone product. This reaction takes place in the presence of a catalyst \[aluminium{\text{ }}chloride\], it first generates an electrophile which gets attached to a benzene ring to give a major product \[4 - methoxyacetophenone\]
In the reaction only major product is shown which is an ortho product. The alkoxy group is always an ortho-para directing in electrophilic aromatic substitution reactions.
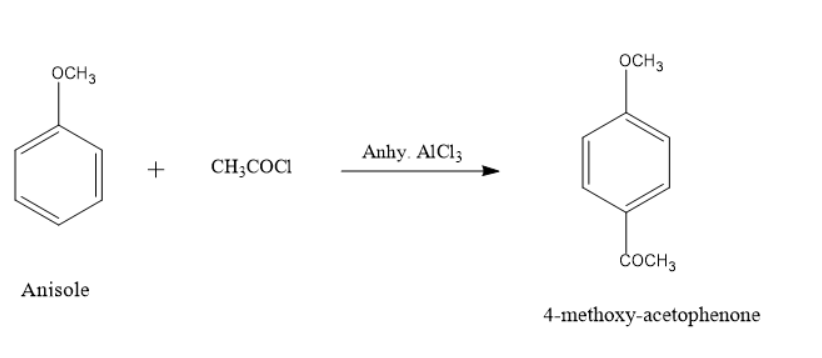
The major product name is \[4 - methoxyacetophenone\] or \[1 - (4 - methoxyphenyl)ethanone\]. Here the functional group is ketone, therefore the suffix \[ - one\]. It is also known as Acetanisole which smells like butter or caramel and has a sweet and fruity smell.
Note:
There are many other examples of acetylation. One of them is acetylation of glucose where bond formation takes place between the extra electrons of nucleophilic \[oxygen\] with the acetyl group, which results in substitution of \[hydrogen\] atoms from phenol group.
Complete Answer:
The process where an acetyl group is added to a compound because of its replacement with that of an active \[hydrogen\] atom is called acetylation. An acetyl group is included in the organic compound, such a compound is termed as acetates or acetate esters. Acetyl group consists of carbonyl atoms or a \[carbon\] atom bonded in double pairs with \[oxygen\] or a methyl group on end.
Now that we know the acetylation process, we can determine the product.
When \[anisole\]reacts with acetyl chloride it yields a ketone product. This reaction takes place in the presence of a catalyst \[aluminium{\text{ }}chloride\], it first generates an electrophile which gets attached to a benzene ring to give a major product \[4 - methoxyacetophenone\]
In the reaction only major product is shown which is an ortho product. The alkoxy group is always an ortho-para directing in electrophilic aromatic substitution reactions.

The major product name is \[4 - methoxyacetophenone\] or \[1 - (4 - methoxyphenyl)ethanone\]. Here the functional group is ketone, therefore the suffix \[ - one\]. It is also known as Acetanisole which smells like butter or caramel and has a sweet and fruity smell.
Note:
There are many other examples of acetylation. One of them is acetylation of glucose where bond formation takes place between the extra electrons of nucleophilic \[oxygen\] with the acetyl group, which results in substitution of \[hydrogen\] atoms from phenol group.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




