
How many isomers are possible for ${C_6}{H_{12}}$?
A: $25$
B: $24$
C: $22$
D: None of these
Answer
585.9k+ views
Hint:Isomers are the molecules or ions that have the same molecular formula, that is they have the same number of atoms of each element but their arrangement is different. Isomers may or may not have the same physical and chemical properties.
Complete step by step answer:
Isomers are the molecules or ions that have the same molecular formula, that is they have the same number of atoms of each element but their arrangement is different. In ${C_6}{H_{12}}$ there are six atoms of carbon and twelve atoms of hydrogen. The possible structures of this compound are as follows:
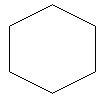
$1$. Cyclohexane: this is a cyclic hydrocarbon in which carbon atoms are arranged in the form of a ring.
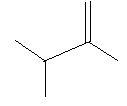
In the following three compounds there is a double bond between two carbon atoms in the main chain and two methyl groups are also present in the main chain.
$2$. $2,3 - $Dimethyl$ - 1 - $butene
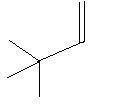
$3$. $3,3 - $Dimethyl$ - 1 - $butene
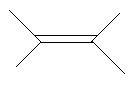
$4$. $2,3 - $Dimethyl$ - 2 - $butene
In the following three compounds there is a ring of four carbon atoms and two methyl groups are present at different positions.
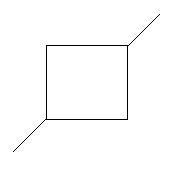
$5$. $1,1 - $Dimethylcyclobutane
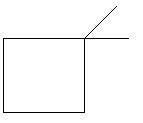
$6$. $1,2 - $Dimethylcyclobutane
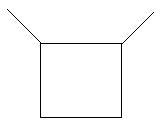
$7$. $1,3 - $Dimethylcyclobutane
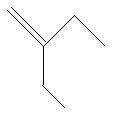
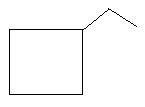
$8$. $2 - $Ethyl$ - 1 - $butene: in this compound there are four carbon atoms in the main chain with a double bond at first carbon atom and one ethyl group is present at second carbon atom.
$9$. Ethylcyclobutane: in this compound there is a ring of four carbon atoms and one ethyl group is present as the first carbon atom of the ring.
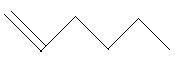
In the following three compounds, there is a main chain of six carbon atoms and one double bond is present at different positions.
$10$. $1 - $Hexene
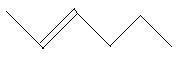
$11$. $2 - $Hexene
$12$. $3 - $Hexene
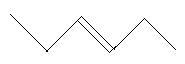
In the following six compounds there is a main chain of five carbon atoms in which methyl groups and position of double bond changes.
$13$. $2 - $Methyl$ - 1 - $pentene
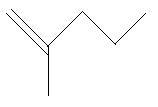
$14$. $2 - $Methyl$ - 2 - $pentene
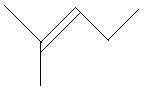
$15$. $3 - $Methyl$ - 1 - $pentene
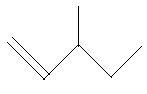
$16$. $3 - $Methyl$ - 2 - $pentene
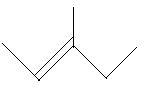
$17$. $4 - $Methyl$ - 1 - $pentene
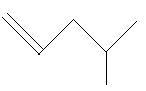
$18$. $4 - $Methyl$ - 2 - $pentene
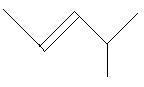
In the following two compounds there is a ring of three carbon atoms and three methyl groups are present at three different positions.
$19$. $1,2,3 - $Trimethylcyclopropane

$20$. $1,1,2 - $Trimethylcyclopropane
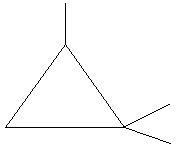
$21$. Propylcyclopropane: in this compound there is a ring of three carbon atoms and a side chain of three carbon atoms is also present.
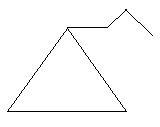
$22$. Methylcyclopentane: in this compound there is a ring of five carbon atoms and one methyl group is attached to this ring.
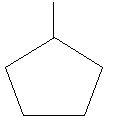
$23$. Isopropylcyclopropane:
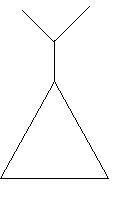
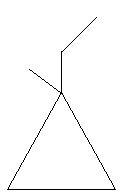
In the following two compounds there is a ring of three carbon atoms and the position of one ethyl and methyl group varies.
$24$. $1 - $Ethyl$ - 1 - $methylcyclopropane
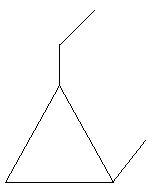
$25$. $1 - $Ethyl$ - 2 - $methylcyclopropane
So, there are total $25$ isomers of ${C_6}{H_{12}}$.
Hence the correct option is A.
Note:
In these isomers geometric isomers and enantiomers are not included. Enantiomers are the non-superimposable images of each other. Enantiomer is one of the two stereoisomers. Stereoisomers are the isomers which differ in three dimensional arrangement.
Complete step by step answer:
Isomers are the molecules or ions that have the same molecular formula, that is they have the same number of atoms of each element but their arrangement is different. In ${C_6}{H_{12}}$ there are six atoms of carbon and twelve atoms of hydrogen. The possible structures of this compound are as follows:
$1$. Cyclohexane: this is a cyclic hydrocarbon in which carbon atoms are arranged in the form of a ring.

In the following three compounds there is a double bond between two carbon atoms in the main chain and two methyl groups are also present in the main chain.
$2$. $2,3 - $Dimethyl$ - 1 - $butene

$3$. $3,3 - $Dimethyl$ - 1 - $butene

$4$. $2,3 - $Dimethyl$ - 2 - $butene

In the following three compounds there is a ring of four carbon atoms and two methyl groups are present at different positions.
$5$. $1,1 - $Dimethylcyclobutane

$6$. $1,2 - $Dimethylcyclobutane

$7$. $1,3 - $Dimethylcyclobutane

$8$. $2 - $Ethyl$ - 1 - $butene: in this compound there are four carbon atoms in the main chain with a double bond at first carbon atom and one ethyl group is present at second carbon atom.

$9$. Ethylcyclobutane: in this compound there is a ring of four carbon atoms and one ethyl group is present as the first carbon atom of the ring.

In the following three compounds, there is a main chain of six carbon atoms and one double bond is present at different positions.
$10$. $1 - $Hexene

$11$. $2 - $Hexene

$12$. $3 - $Hexene

In the following six compounds there is a main chain of five carbon atoms in which methyl groups and position of double bond changes.
$13$. $2 - $Methyl$ - 1 - $pentene

$14$. $2 - $Methyl$ - 2 - $pentene

$15$. $3 - $Methyl$ - 1 - $pentene

$16$. $3 - $Methyl$ - 2 - $pentene

$17$. $4 - $Methyl$ - 1 - $pentene

$18$. $4 - $Methyl$ - 2 - $pentene

In the following two compounds there is a ring of three carbon atoms and three methyl groups are present at three different positions.
$19$. $1,2,3 - $Trimethylcyclopropane

$20$. $1,1,2 - $Trimethylcyclopropane

$21$. Propylcyclopropane: in this compound there is a ring of three carbon atoms and a side chain of three carbon atoms is also present.

$22$. Methylcyclopentane: in this compound there is a ring of five carbon atoms and one methyl group is attached to this ring.

$23$. Isopropylcyclopropane:

In the following two compounds there is a ring of three carbon atoms and the position of one ethyl and methyl group varies.
$24$. $1 - $Ethyl$ - 1 - $methylcyclopropane

$25$. $1 - $Ethyl$ - 2 - $methylcyclopropane

So, there are total $25$ isomers of ${C_6}{H_{12}}$.
Hence the correct option is A.
Note:
In these isomers geometric isomers and enantiomers are not included. Enantiomers are the non-superimposable images of each other. Enantiomer is one of the two stereoisomers. Stereoisomers are the isomers which differ in three dimensional arrangement.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




