
How many isomeric vicinal-dihalides are possible for the compound having a molecular formula \[{C_3}{H_6}C{l_2}\]?
A.1
B.2
C.3
D.4
Answer
509.1k+ views
Hint: Vicinal compounds have halogens on adjacent carbons. Elaborate the molecular formula to carefully look at the structure and then decide the correct answer to the problem.
Complete answer:
In the question it is asked to identify how many isomeric vicinal-dihalides are possible for the compound \[{C_3}{H_6}C{l_2}\]. For this you should know what vicinal dihalides are. So vicinal dihalides are those compounds which have halogens present on the adjacent carbons. The vicinal dihalides are obtained as a result of two successive elimination reactions.
Now we will look into the structure from the molecular formula:\[{C_3}{H_6}C{l_2}\] The structure is:
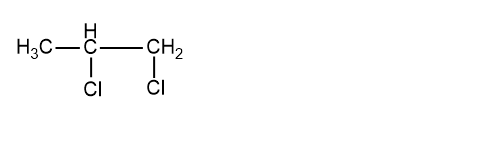
The name of the above compound is 1, 2-dichloropropane. In this structure, we can see that two adjacent carbons are having Cl halogen attach to them so this is a vicinal dihalide. Let us now try to make an isomer of this compound to see whether it is possible or not. So the isomer of this is:
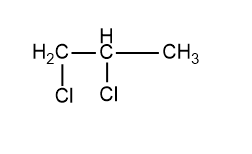
This is the isomer that we get but the name of this compound is also 1,2-dichloropropane, both the compounds drawn above and below are the same. No more isomeric vicinal halides can be drawn for this compound. Hence only 1 isomeric vicinal dihalide is possible for the molecular formula \[{C_3}{H_6}C{l_2}\]
Therefore the correct option \[A.{\text{ }}1\].
Note:
There are also geminal-dihalides. These compounds have halogens present on the same carbon. They also are the result of two successive elimination reactions. Both vicinal-dihalide and geminal-dihalides are important concepts in chemistry.
Complete answer:
In the question it is asked to identify how many isomeric vicinal-dihalides are possible for the compound \[{C_3}{H_6}C{l_2}\]. For this you should know what vicinal dihalides are. So vicinal dihalides are those compounds which have halogens present on the adjacent carbons. The vicinal dihalides are obtained as a result of two successive elimination reactions.
Now we will look into the structure from the molecular formula:\[{C_3}{H_6}C{l_2}\] The structure is:

The name of the above compound is 1, 2-dichloropropane. In this structure, we can see that two adjacent carbons are having Cl halogen attach to them so this is a vicinal dihalide. Let us now try to make an isomer of this compound to see whether it is possible or not. So the isomer of this is:

This is the isomer that we get but the name of this compound is also 1,2-dichloropropane, both the compounds drawn above and below are the same. No more isomeric vicinal halides can be drawn for this compound. Hence only 1 isomeric vicinal dihalide is possible for the molecular formula \[{C_3}{H_6}C{l_2}\]
Therefore the correct option \[A.{\text{ }}1\].
Note:
There are also geminal-dihalides. These compounds have halogens present on the same carbon. They also are the result of two successive elimination reactions. Both vicinal-dihalide and geminal-dihalides are important concepts in chemistry.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




