
Is $Z{{n}^{2+}}$ Paramagnetic or Diamagnetic?
Answer
540.3k+ views
Hint: Paramagnetic and Diamagnetic are the characteristics of the compound which tell the magnetic effect of the compound. If there is an unpaired electron in the compound then it will behave paramagnetically, else it will be diamagnetic. $Z{{n}^{2+}}$ means there will be a loss of 2 electrons from a total number of electrons in it.
Complete step-by-step answer:The magnetic behavior of the compound tells how the compound will behave in the external magnetic field. Paramagnetic and Diamagnetic are the characteristics of the compound which tell the magnetic effect of the compound. We can tell whether the compound or element is paramagnetic or diamagnetic by observing the number of electrons in it.
If there is an unpaired electron in the compound then it will behave paramagnetically, else it will be diamagnetic.
Zinc (Zn) is the element of group 12 in the d-block and the period is 4, so its atomic number will be 30. Therefore, there are 30 electrons in the zinc. The electronic configuration will be:
$1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}3{{s}^{2}}3{{p}^{6}}4{{s}^{2}}3{{d}^{10}}$
Now, the given form of zinc $Z{{n}^{2+}}$ means there will be a loss of 2 electrons from the total number of electrons in it. So, the number of electrons will be 28. The electronic configuration will be:
$1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}3{{s}^{2}}3{{p}^{6}}3{{d}^{10}}$
So, let us see the arrangement of electrons in 3d:
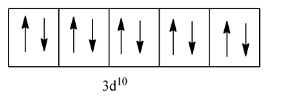
As we can see that there is no unpaired electron in this element, so it will be a diamagnetic element.
Therefore, $Z{{n}^{2+}}$ is a diamagnetic element.
Note: Diamagnetic means that when the zinc is placed in the external magnetic field, it will be weakly magnetized, and paramagnetic means that when the compound is placed in the external magnetic field, it will be strongly magnetized.
Complete step-by-step answer:The magnetic behavior of the compound tells how the compound will behave in the external magnetic field. Paramagnetic and Diamagnetic are the characteristics of the compound which tell the magnetic effect of the compound. We can tell whether the compound or element is paramagnetic or diamagnetic by observing the number of electrons in it.
If there is an unpaired electron in the compound then it will behave paramagnetically, else it will be diamagnetic.
Zinc (Zn) is the element of group 12 in the d-block and the period is 4, so its atomic number will be 30. Therefore, there are 30 electrons in the zinc. The electronic configuration will be:
$1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}3{{s}^{2}}3{{p}^{6}}4{{s}^{2}}3{{d}^{10}}$
Now, the given form of zinc $Z{{n}^{2+}}$ means there will be a loss of 2 electrons from the total number of electrons in it. So, the number of electrons will be 28. The electronic configuration will be:
$1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}3{{s}^{2}}3{{p}^{6}}3{{d}^{10}}$
So, let us see the arrangement of electrons in 3d:

As we can see that there is no unpaired electron in this element, so it will be a diamagnetic element.
Therefore, $Z{{n}^{2+}}$ is a diamagnetic element.
Note: Diamagnetic means that when the zinc is placed in the external magnetic field, it will be weakly magnetized, and paramagnetic means that when the compound is placed in the external magnetic field, it will be strongly magnetized.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




