
Is $S{F_4}$ molecule polar or nonpolar?
Answer
495.3k+ views
Hint: To determine whether the given compound is polar or nonpolar, we first need to sketch its Lewis structure for the given compound and then, if there are no polar bonds in a molecule, then the molecule is considered as nonpolar whereas if molecule possess a polar bond existing without a symmetry, then the molecule is said to be polar.
Complete answer:
To predict the polarity of a molecule, the following general steps are needed to be followed:
Step-1: Sketch a reasonable Lewis structure of the given molecule i.e., $S{F_4}$:
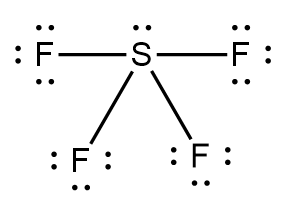
Step-2: Identify each bond in the structure as either polar or nonpolar. If the electronegativity difference between the bonds is greater than $0.4$, then the bond is considered as polar while if the electronegativity difference is less than $0.4$, then the bond is considered as nonpolar.
In $S{F_4}$, the electronegativity difference between the $S - F$ bond is $2.08$ which is greater than $0.4$. Thus, bonds in $S{F_4}$ are polar.
Step-3: If the bonds in the given molecule are polar then draw the geometric sketch of the molecule. The geometric sketch of $S{F_4}$ molecule is as follows:
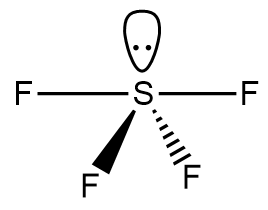
Step-4: Determine the symmetry of the molecule as if the arrangement is symmetrical and arrows balance each other, then the molecule is nonpolar whereas if the arrangement is asymmetrical, then the molecule is polar.
As per the geometric sketch of the $S{F_4}$ molecule, it exists in a see-saw geometry and the sulphur atom consists of a lone pair of electrons which makes the molecule asymmetrical. Thus, we can conclude that the $S{F_4}$ molecule is polar in nature.
Note:
It is important to note that the Lewis structure of a molecule may give a false impression of the geometry of the molecule they represent. So, the Lewis structures are used to determine individual bond polarity while the geometry of the molecule predicts the polarity of the overall molecule.
Complete answer:
To predict the polarity of a molecule, the following general steps are needed to be followed:
Step-1: Sketch a reasonable Lewis structure of the given molecule i.e., $S{F_4}$:

Step-2: Identify each bond in the structure as either polar or nonpolar. If the electronegativity difference between the bonds is greater than $0.4$, then the bond is considered as polar while if the electronegativity difference is less than $0.4$, then the bond is considered as nonpolar.
In $S{F_4}$, the electronegativity difference between the $S - F$ bond is $2.08$ which is greater than $0.4$. Thus, bonds in $S{F_4}$ are polar.
Step-3: If the bonds in the given molecule are polar then draw the geometric sketch of the molecule. The geometric sketch of $S{F_4}$ molecule is as follows:

Step-4: Determine the symmetry of the molecule as if the arrangement is symmetrical and arrows balance each other, then the molecule is nonpolar whereas if the arrangement is asymmetrical, then the molecule is polar.
As per the geometric sketch of the $S{F_4}$ molecule, it exists in a see-saw geometry and the sulphur atom consists of a lone pair of electrons which makes the molecule asymmetrical. Thus, we can conclude that the $S{F_4}$ molecule is polar in nature.
Note:
It is important to note that the Lewis structure of a molecule may give a false impression of the geometry of the molecule they represent. So, the Lewis structures are used to determine individual bond polarity while the geometry of the molecule predicts the polarity of the overall molecule.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




