
Is ${{H}_{2}}O$ show dipole-dipole interaction?
Answer
541.5k+ views
Hint:Dipole-dipole interaction is a type of interaction between the elements in a compound. Dipole-dipole interactions are only formed by those compounds which are polar, i.e., there is a difference in the atoms of the compound which creates polarity in the molecule.
Complete step-by-step answer:Dipole-dipole interaction is an intermolecular force existing among the molecules. This interaction is only formed in those compounds in which the polarity is present, i.e., those compounds which are polar. Polar molecules are those compounds in which the elements joined in the compound have different electronegativity. Like the HCl compound, the electronegativity of Hydrogen is very small while the electronegativity of chlorine is high. This creates a polarity towards the more electronegative atom.
So, a water molecule contains hydrogen and oxygen atoms, and oxygen is also an element that has high electronegativity. Therefore, there will be a difference in the electronegativities. Water is a polar molecule. The polarity in water is given below:
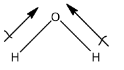
The dipole-dipole interaction is formed because the negative part of the molecule will attract the positive part of the other molecule. In water, the oxygen has a slight negative charge that will try to attract the hydrogen element of another water molecule. This interaction is water is dipole-dipole interaction.
Therefore, the given statement in the question is true, i.e., the water has dipole-dipole interaction.
Note:There are many more types of interaction like ion-dipole interaction, ion-induced dipole interaction, etc. The ion-dipole interaction is formed when the salts are dissolved in water, and ion-induced dipole interaction is formed between $NO_{3}^{-}$ ion and ${{I}_{2}}$.
Complete step-by-step answer:Dipole-dipole interaction is an intermolecular force existing among the molecules. This interaction is only formed in those compounds in which the polarity is present, i.e., those compounds which are polar. Polar molecules are those compounds in which the elements joined in the compound have different electronegativity. Like the HCl compound, the electronegativity of Hydrogen is very small while the electronegativity of chlorine is high. This creates a polarity towards the more electronegative atom.
So, a water molecule contains hydrogen and oxygen atoms, and oxygen is also an element that has high electronegativity. Therefore, there will be a difference in the electronegativities. Water is a polar molecule. The polarity in water is given below:

The dipole-dipole interaction is formed because the negative part of the molecule will attract the positive part of the other molecule. In water, the oxygen has a slight negative charge that will try to attract the hydrogen element of another water molecule. This interaction is water is dipole-dipole interaction.
Therefore, the given statement in the question is true, i.e., the water has dipole-dipole interaction.
Note:There are many more types of interaction like ion-dipole interaction, ion-induced dipole interaction, etc. The ion-dipole interaction is formed when the salts are dissolved in water, and ion-induced dipole interaction is formed between $NO_{3}^{-}$ ion and ${{I}_{2}}$.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

What organs are located on the left side of your body class 11 biology CBSE

Draw a diagram of nephron and explain its structur class 11 biology CBSE




