
Is $CC{{l}_{4}}$ covalent or ionic?
Answer
541.5k+ views
Hint: Ionic and covalent are two types of bonds formed between the atoms in a molecule. Ionic and covalent bonds depend on the nature of the4 atoms, i.e., metal element or non-metal element. If one is metal and the other is non-metal, then it is an ionic bond, and if both of them are non-metals, then it is a covalent bond.
Complete step-by-step answer: Ionic and covalent are two types of bonds formed between the atoms in a molecule. Ionic and covalent bonds depend on the nature of the4 atoms, i.e., metal element or non-metal element. If one is metal and the other is non-metal, then it is an ionic bond, and if both of them are non-metals, then it is a covalent bond.
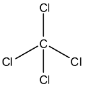
More specifically speaking, carbon tetrachloride is a nonpolar covalent compound because the electrons shared by the carbon and chlorine atoms are nearly at the center of the bond.
Therefore, the carbon tetrachloride ($CC{{l}_{4}}$) is a covalent compound.
Note:Other examples of nonpolar covalent bonds are ${{N}_{2}}$, ${{O}_{2}}$, $C{{l}_{2}}$, etc. and some examples of the polar covalent bond are hydrochloric acid (HCl), bromine chloride (BrCl), water (${{H}_{2}}O$), etc.
Complete step-by-step answer: Ionic and covalent are two types of bonds formed between the atoms in a molecule. Ionic and covalent bonds depend on the nature of the4 atoms, i.e., metal element or non-metal element. If one is metal and the other is non-metal, then it is an ionic bond, and if both of them are non-metals, then it is a covalent bond.

More specifically speaking, carbon tetrachloride is a nonpolar covalent compound because the electrons shared by the carbon and chlorine atoms are nearly at the center of the bond.
Therefore, the carbon tetrachloride ($CC{{l}_{4}}$) is a covalent compound.
Note:Other examples of nonpolar covalent bonds are ${{N}_{2}}$, ${{O}_{2}}$, $C{{l}_{2}}$, etc. and some examples of the polar covalent bond are hydrochloric acid (HCl), bromine chloride (BrCl), water (${{H}_{2}}O$), etc.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




