
Increasing order of rate with $HN{O_3}/{H_2}S{O_4}$ is?
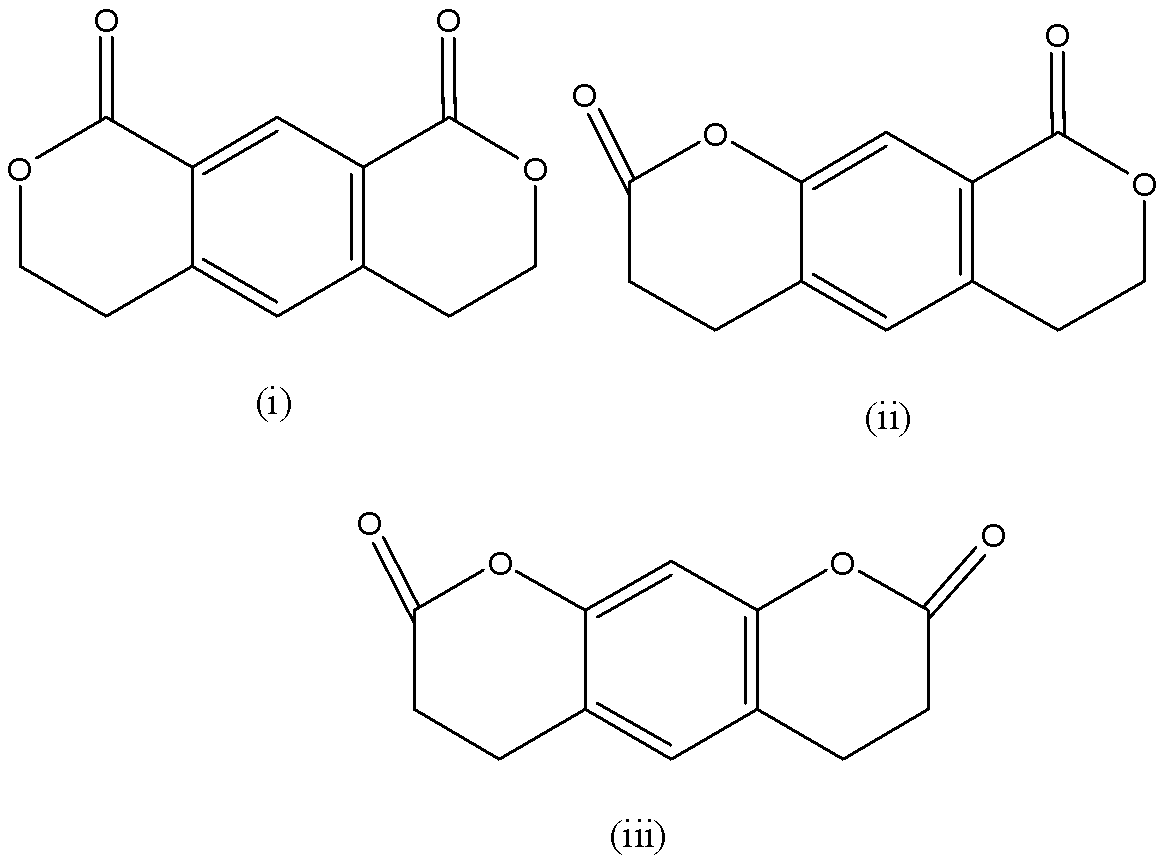
A) \[\left( {iii} \right) < \left( {ii} \right) < \left( i \right)\].
B) \[\left( {ii} \right) < \left( {iii} \right) < \left( i \right)\].
C) \[\left( {i} \right) < \left( {iii} \right) < \left( {ii} \right)\].
D) \[\left( {i} \right) < \left( {ii} \right) < \left( {iii} \right)\].

Answer
582.3k+ views
Hint: We know that the reaction order towards \[HN{O_3}\] varied with \[{H_2}S{O_4}\] concentration. In lower concentrations of \[{H_2}S{O_4}\], the reaction rate increases with increase of \[HN{O_3}\]. In high \[{H_2}S{O_4}\] concentration media, though, zero order reaction is observed towards \[HN{O_3}\].
Complete step by step answer:We must remember in this reaction the compounds are treated with a mix of concentrated $HN{O_3}/{H_2}S{O_4}$ at a temperature not exceeding that \[{50^o}C\]. The dissociation of these acids are given below as,
\[HN{O_3} + {H_{2{\text{ }}}}S{O_4}{\text{ }} \to N{O_2}{\text{ }} + 2HS{O_4}^ - {\text{ }} + {H_3}{O^ + }\]
The increasing order of rate of reaction is \[\left( {iii} \right) < \left( {ii} \right) < \left( i \right)\].
Therefore, the option A is correct.
This is due to the fact these compounds will undergo nitration reaction when they react with nitric acid or sulphuric acid. Generally the $N{O_2}$ attacks the position or a compound which has lesser amount of sterically hindrance and can easily donate electrons.
Note:In nitromethane, Electron withdrawing groups are present and electron withdrawing is a Nitro group. During this molecule \[N{O_2}\] has partial charge since it attracts the charge density towards its side. Methyl group has partial charge since it suffers from partial loss of charge density. In the case of electron donating groups it is sort of different. Alkyl groups, alkoxy groups belong to the present category. Within the case of toluene (methyl is attached to benzene) the methyl present is electron donating. It donates the electron density to benzene formula.
Complete step by step answer:We must remember in this reaction the compounds are treated with a mix of concentrated $HN{O_3}/{H_2}S{O_4}$ at a temperature not exceeding that \[{50^o}C\]. The dissociation of these acids are given below as,
\[HN{O_3} + {H_{2{\text{ }}}}S{O_4}{\text{ }} \to N{O_2}{\text{ }} + 2HS{O_4}^ - {\text{ }} + {H_3}{O^ + }\]
The increasing order of rate of reaction is \[\left( {iii} \right) < \left( {ii} \right) < \left( i \right)\].
Therefore, the option A is correct.
This is due to the fact these compounds will undergo nitration reaction when they react with nitric acid or sulphuric acid. Generally the $N{O_2}$ attacks the position or a compound which has lesser amount of sterically hindrance and can easily donate electrons.
Note:In nitromethane, Electron withdrawing groups are present and electron withdrawing is a Nitro group. During this molecule \[N{O_2}\] has partial charge since it attracts the charge density towards its side. Methyl group has partial charge since it suffers from partial loss of charge density. In the case of electron donating groups it is sort of different. Alkyl groups, alkoxy groups belong to the present category. Within the case of toluene (methyl is attached to benzene) the methyl present is electron donating. It donates the electron density to benzene formula.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




