
In which of the following pairs, indicated bond is of greater strength:
A.

B.
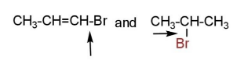
C.
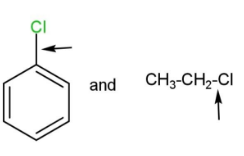
D.
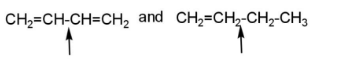
E.
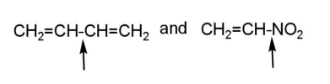
F.



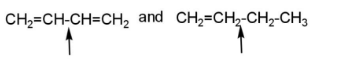
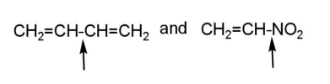

Answer
533.7k+ views
Hint: When the atoms are held together in a molecule, bonds are formed due to their interactions. These bonds are of varied lengths and strengths.Bond order is responsible for the length of any bond, and length of any bond has an effect on the bond strength.
Complete answer:
Bonds are the species that link together atoms. These bonds are formed by either sharing of electrons between atoms (in covalent bond) or by transfer of electrons (in ionic bond).
As we know, length of a bond is defined as the distance between the nuclei of two atoms bonded together. While, Bond order is the number of bonds between the atoms. If the bond order is high, then the bond length is shorter, so, the bond energy is also high, as energy is required to break more bonds, which means that the bond strength is greater. So, shorter bond lengths have higher energy requirements for their breaking. Therefore, this will increase the bond strength in atoms with high bond order.
Also, the atoms that have delocalization, and hyper conjugation, due to double or triple bonds have higher bond strength. This is due to the fact that bond order and bond length are related, more electron pairs result in short bonds and high bond strength. Thus, bond length is inversely proportional to bond strength.
Through these facts, it can be inferred that pairs B, D, E, and F have compounds in which greater bond strength is indicated, due to more bond order and less bond length, also due to hyper conjugation.
Some of the compounds that have more bond strength in these pairs are:
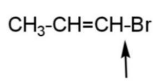
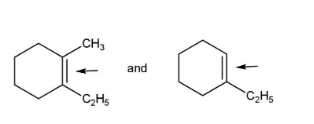
pair B has hyper conjugation due to more bond order, and double bond that decreases bond length and increases bond strength.
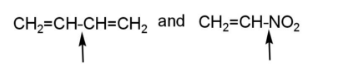
pair E has both the indicated bonds with greater strength due to the double bond character and more bond order, because of hyper conjugation.
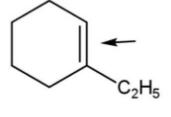
compound from pair F, has less carbon chain so shorter bond length so, more bond strength.
Pairs, D have only the first compound with more bond strength due to the same reasons.
Hence, pairs B, D, E, and F have compounds indicating greater bond strength.
Note: Hybridization also affects the bond length, and hence the bond strength, more s character results in a longer length and less bond strength, while a decreased s character has more bond strength.
Complete answer:
Bonds are the species that link together atoms. These bonds are formed by either sharing of electrons between atoms (in covalent bond) or by transfer of electrons (in ionic bond).
As we know, length of a bond is defined as the distance between the nuclei of two atoms bonded together. While, Bond order is the number of bonds between the atoms. If the bond order is high, then the bond length is shorter, so, the bond energy is also high, as energy is required to break more bonds, which means that the bond strength is greater. So, shorter bond lengths have higher energy requirements for their breaking. Therefore, this will increase the bond strength in atoms with high bond order.
Also, the atoms that have delocalization, and hyper conjugation, due to double or triple bonds have higher bond strength. This is due to the fact that bond order and bond length are related, more electron pairs result in short bonds and high bond strength. Thus, bond length is inversely proportional to bond strength.
Through these facts, it can be inferred that pairs B, D, E, and F have compounds in which greater bond strength is indicated, due to more bond order and less bond length, also due to hyper conjugation.
Some of the compounds that have more bond strength in these pairs are:
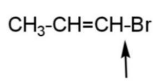
pair B has hyper conjugation due to more bond order, and double bond that decreases bond length and increases bond strength.
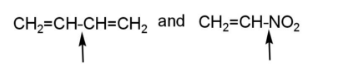
pair E has both the indicated bonds with greater strength due to the double bond character and more bond order, because of hyper conjugation.

compound from pair F, has less carbon chain so shorter bond length so, more bond strength.
Pairs, D have only the first compound with more bond strength due to the same reasons.
Hence, pairs B, D, E, and F have compounds indicating greater bond strength.
Note: Hybridization also affects the bond length, and hence the bond strength, more s character results in a longer length and less bond strength, while a decreased s character has more bond strength.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

What organs are located on the left side of your body class 11 biology CBSE

Draw a labelled diagram of the human heart and label class 11 biology CBSE

What is 1s 2s 2p 3s 3p class 11 chemistry CBSE




