
In which case, the carbon-carbon bond length is the same?
(A) 2-butene
(B) Benzene
(C) 1-butene
(D) 1-propyne
Answer
582.3k+ views
Hint: The Carbon-Carbon bond length of single bond is 154pm, and Carbon-Carbon bond length of double bond is 134pm. The bond length of the carbon-carbon triple bond is 120 pm . Conjugation decreases the bond length of a single bond and increases the bond length of double bond.
Complete step by step answer:
To see which of these options have all the same carbon-carbon bond length, we will draw the structure of each compound.
Considering option (A), 2-butene. The structure of 2-butene is as follows:
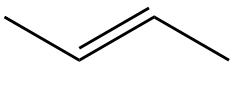
In this compound we can see that there is one double bond and two single bonds, therefore, all carbon-carbon bond lengths will not be the same. Therefore, it is not a correct option.
Considering option (B), Benzene. The structure of Benzene is as follows:
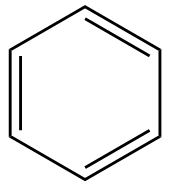
In this compound we can see that, there are 3 double bonds, there are 3 single bonds. Bond lengths are all same, instead of presence of single and bonds, because the double are in resonance, therefore, bonds are interchanged between two carbon atoms.The resonance structures of benzene is as follows:
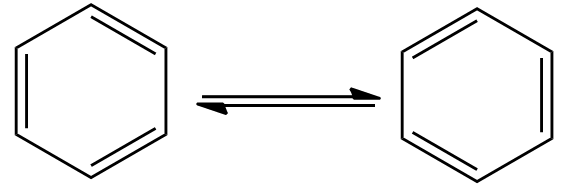
Therefore, each carbon-carbon bond is of the same length that is 140pm. Therefore, it is the correct option.
Considering option (C), 1-butene. The structure of 1-butene is as follows:

The compound 1-butene has one double bond and two single bonds therefore, the bond lengths of C-C are not equal.
Considering option (D), 1-propyne. The structure of 1-propyne is as follows:
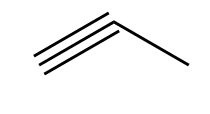
This compound has one triple bond and one single bond, so it also does not have the same C-C bond length.
So, the correct answer is “Option B”.
Note: Resonance results in partial double bond character in the molecule and hence increases the bond strength of single bonds that is the bond length after resonance decreases, and similarly decreases the bond strength of double bond and bond length increases.
Complete step by step answer:
To see which of these options have all the same carbon-carbon bond length, we will draw the structure of each compound.
Considering option (A), 2-butene. The structure of 2-butene is as follows:

In this compound we can see that there is one double bond and two single bonds, therefore, all carbon-carbon bond lengths will not be the same. Therefore, it is not a correct option.
Considering option (B), Benzene. The structure of Benzene is as follows:

In this compound we can see that, there are 3 double bonds, there are 3 single bonds. Bond lengths are all same, instead of presence of single and bonds, because the double are in resonance, therefore, bonds are interchanged between two carbon atoms.The resonance structures of benzene is as follows:

Therefore, each carbon-carbon bond is of the same length that is 140pm. Therefore, it is the correct option.
Considering option (C), 1-butene. The structure of 1-butene is as follows:

The compound 1-butene has one double bond and two single bonds therefore, the bond lengths of C-C are not equal.
Considering option (D), 1-propyne. The structure of 1-propyne is as follows:

This compound has one triple bond and one single bond, so it also does not have the same C-C bond length.
So, the correct answer is “Option B”.
Note: Resonance results in partial double bond character in the molecule and hence increases the bond strength of single bonds that is the bond length after resonance decreases, and similarly decreases the bond strength of double bond and bond length increases.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




