
In tris(ethylenediamine) cobalt (III) chloride, find the coordination number of cobalt.
Answer
582.6k+ views
Hint: We can say the coordination number of the central metal ion (or) atom is the number of donor atoms linked to it.
Complete step by step answer:
As we know that the coordination complex is a compound that consists of a central atom (ion) that is metallic and is known as the coordination centre. It is enclosed by array bound molecules (or) ions called ligands (or) complexing agents. Transition metals are coordination complexes. A coordination complex whose centre contains a metal atom is known as a metal complex of d block element.
Tris (ethylenediamine) cobalt (III) chloride is an example of inorganic compound. We can write the formula of tris(ethylenediamine) cobalt (III) chloride as $\left[ {Co{{\left( {en} \right)}_3}} \right]C{l_3}$. We can abbreviate $en$ as ethylenediamine. An chloride salt of the coordination complex ${\left[ {Co{{\left( {en} \right)}_3}} \right]^{3 + }}$ is tris (ethylenediamine) cobalt (III) chloride.
We can draw the structure of tris (ethylenediamine) cobalt (III) chloride as,
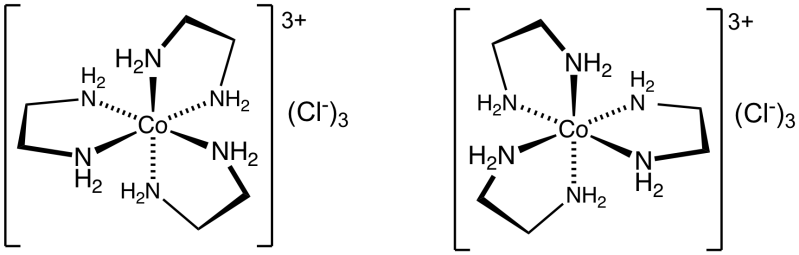
We can define coordination number as the number of ligand donor atoms to which central metal/ion is straightly linked in a complex/coordination compound.
We know that Ethane—1,2-diamine (or) ethylene diamine is a bidentate ligand and it provides two donor atoms. The prefix tris indicates three atoms of ethylene diamine.
Therefore, the coordination number of cobalt would be $3 \times 2 = 6$. In the complex, ${\left[ {Co{{\left( {en} \right)}_3}} \right]^{3 + }}$ the coordination number of cobalt would be six.
Note:
We must remember that the coordination compounds contain specific colours. Therefore, they are used in industries for intense colourations. Phthalocyanine is a class of coordination complexes that the dyes and pigments industry use.
EDTA is another complex compound that is used for determining the hardness of water. Coordination compounds could also be used as catalysts. These days, they are used in the polymer industry. Coordination compounds can be used in the extraction of metals from their ores. Extraction of nickel and cobalt by hydro-metallurgical processes requires a lot of complex ions.
Complete step by step answer:
As we know that the coordination complex is a compound that consists of a central atom (ion) that is metallic and is known as the coordination centre. It is enclosed by array bound molecules (or) ions called ligands (or) complexing agents. Transition metals are coordination complexes. A coordination complex whose centre contains a metal atom is known as a metal complex of d block element.
Tris (ethylenediamine) cobalt (III) chloride is an example of inorganic compound. We can write the formula of tris(ethylenediamine) cobalt (III) chloride as $\left[ {Co{{\left( {en} \right)}_3}} \right]C{l_3}$. We can abbreviate $en$ as ethylenediamine. An chloride salt of the coordination complex ${\left[ {Co{{\left( {en} \right)}_3}} \right]^{3 + }}$ is tris (ethylenediamine) cobalt (III) chloride.
We can draw the structure of tris (ethylenediamine) cobalt (III) chloride as,

We can define coordination number as the number of ligand donor atoms to which central metal/ion is straightly linked in a complex/coordination compound.
We know that Ethane—1,2-diamine (or) ethylene diamine is a bidentate ligand and it provides two donor atoms. The prefix tris indicates three atoms of ethylene diamine.
Therefore, the coordination number of cobalt would be $3 \times 2 = 6$. In the complex, ${\left[ {Co{{\left( {en} \right)}_3}} \right]^{3 + }}$ the coordination number of cobalt would be six.
Note:
We must remember that the coordination compounds contain specific colours. Therefore, they are used in industries for intense colourations. Phthalocyanine is a class of coordination complexes that the dyes and pigments industry use.
EDTA is another complex compound that is used for determining the hardness of water. Coordination compounds could also be used as catalysts. These days, they are used in the polymer industry. Coordination compounds can be used in the extraction of metals from their ores. Extraction of nickel and cobalt by hydro-metallurgical processes requires a lot of complex ions.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




