
In thiosulphuric acid:
A.Each sulphur atom is in identical oxidation state
B.There is a S = S linkage present
C.One atom is in +2 and other sulphur atom is in +4 oxidation state
D.There is only one replaceable hydrogen atom
Answer
582.3k+ views
Hint: To solve this question, you must follow these steps: first, draw the molecular structure of thiosulphuric acid. On the basis of this diagram, solve for each option given. Only one of these statements is true and upon detailed observation, you would be able to find it.
Complete step by step answer:
Before we move forward with the solution of this question, let us f=draw the molecular structure of thiosulphuric acid:
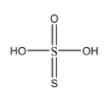
Now, to determine the correct statement about thiosulphuric acid from the given options, let us discuss them:
Each sulphur atom is in identical oxidation state:
The central sulphur atom has formed 4 sigma bonds and 2 pi bonds with the neighbouring atoms. On the other hand, the other sulphur atom has formed only one sigma and one pi bond. Hence the oxidation states of both the sulphur atoms are different.
There is a S = S linkage present:
As we can see in the molecular structure, there is indeed a double bod present between the two sulphur atoms.
One atom is in +2 and other sulphur atom is in +4 oxidation state:
The electronic configuration of sulphur is \[1{s^2}2{s^2}2{p^6}3{s^2}3{p^4}\] . Now, from point 2, we can say that the central sulphur shares with all the 6 electrons present in the valence shell and achieves +6 oxidation state. While the other sulphur atom accepts the 2 electrons shared by the central sulphur atom and achieves an oxidation state of (-2).
There is only one replaceable hydrogen atom:
There are 2 \[ - OH\] groups present in this compound. Each \[ - OH\] group has one replaceable hydrogen. Hence the compound has 2 replaceable hydrogens.
From the above explanations, we can determine that: In thiosulphuric acid there is a S = S linkage present.
Hence, Option B is the correct option.
Note:
Thiosulphuric acid is a sulphur oxoacid. The acid cannot be made by acidifying aqueous thiosulfate salt solutions as the acid readily decomposes in water.
Complete step by step answer:
Before we move forward with the solution of this question, let us f=draw the molecular structure of thiosulphuric acid:

Now, to determine the correct statement about thiosulphuric acid from the given options, let us discuss them:
Each sulphur atom is in identical oxidation state:
The central sulphur atom has formed 4 sigma bonds and 2 pi bonds with the neighbouring atoms. On the other hand, the other sulphur atom has formed only one sigma and one pi bond. Hence the oxidation states of both the sulphur atoms are different.
There is a S = S linkage present:
As we can see in the molecular structure, there is indeed a double bod present between the two sulphur atoms.
One atom is in +2 and other sulphur atom is in +4 oxidation state:
The electronic configuration of sulphur is \[1{s^2}2{s^2}2{p^6}3{s^2}3{p^4}\] . Now, from point 2, we can say that the central sulphur shares with all the 6 electrons present in the valence shell and achieves +6 oxidation state. While the other sulphur atom accepts the 2 electrons shared by the central sulphur atom and achieves an oxidation state of (-2).
There is only one replaceable hydrogen atom:
There are 2 \[ - OH\] groups present in this compound. Each \[ - OH\] group has one replaceable hydrogen. Hence the compound has 2 replaceable hydrogens.
From the above explanations, we can determine that: In thiosulphuric acid there is a S = S linkage present.
Hence, Option B is the correct option.
Note:
Thiosulphuric acid is a sulphur oxoacid. The acid cannot be made by acidifying aqueous thiosulfate salt solutions as the acid readily decomposes in water.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




