
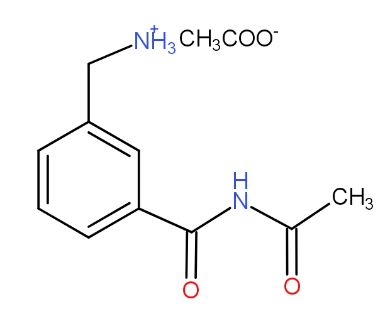
In the reaction shown below, the major product(s) formed is/are :
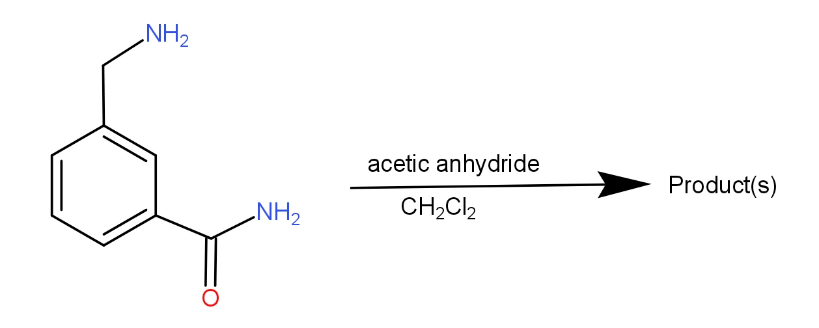
a.)
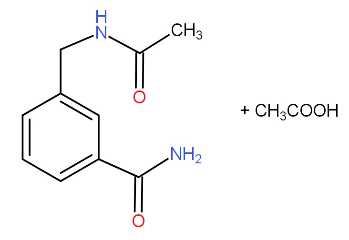
b.)
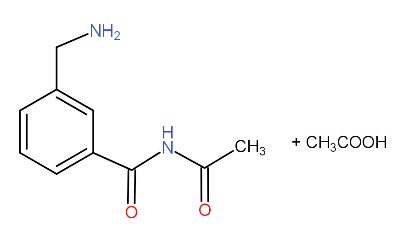
c.)
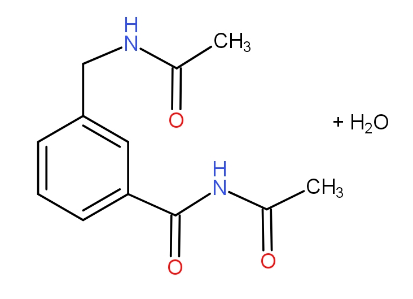
d.)





Answer
579.6k+ views
Hint: This is an acetylation reaction where the amine combines with the acetic anhydride to give acetylated product. The reaction can take place with amine only and not with amide. So, the product will be one that will have amine part acetylated not amide.
Complete step by step answer:
The reagents given to us are acetic anhydride and dichloromethane. The substrate for the reaction has an amine group.
The amine group combines with acetic anhydride to form the product. Such a reaction is called acetylation reaction. As a result, the acetyl group from the acetic anhydride gets attached on the Nitrogen of amine giving the major product along with a molecule of acetic acid.d
The reaction can be written as -
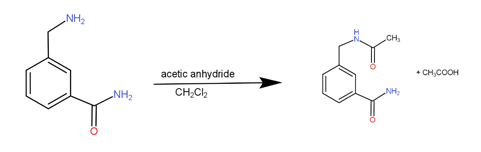
So, the correct answer is “Option A”.
Additional Information:
For acetylation, acetic anhydride and acetyl chloride; any of these reagents can be used. Both the reagents should be used carefully because both can cause irritation. In case, we use acetic anhydride; we get acetic acid along with the product. But with use of acetyl chloride, we get HCl gas. The advantage of use of acetyl chloride is that the by product is HCl gas which can be evolved out.
Note: It must be noted that the n- acetylation is used in organic chemistry to attach an acetyl functional group on an amine compound. The acetyl group can be used as a protecting group in peptide synthesis and many other reactions.
Complete step by step answer:
The reagents given to us are acetic anhydride and dichloromethane. The substrate for the reaction has an amine group.
The amine group combines with acetic anhydride to form the product. Such a reaction is called acetylation reaction. As a result, the acetyl group from the acetic anhydride gets attached on the Nitrogen of amine giving the major product along with a molecule of acetic acid.d
The reaction can be written as -

So, the correct answer is “Option A”.
Additional Information:
For acetylation, acetic anhydride and acetyl chloride; any of these reagents can be used. Both the reagents should be used carefully because both can cause irritation. In case, we use acetic anhydride; we get acetic acid along with the product. But with use of acetyl chloride, we get HCl gas. The advantage of use of acetyl chloride is that the by product is HCl gas which can be evolved out.
Note: It must be noted that the n- acetylation is used in organic chemistry to attach an acetyl functional group on an amine compound. The acetyl group can be used as a protecting group in peptide synthesis and many other reactions.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




