
In the reaction sequence.
\[Cyclohexane\xrightarrow{{hv/C{l_2}}}\left( X \right)\xrightarrow{{alc.KOH/\vartriangle }}\left( Y \right)\xrightarrow[{\dfrac{{{H_2}O}}{{Zn}}}]{{{O_3}}}\left( Z \right)\]will be:
A) Hexanal.
B) 2-hexanone.
C) 3-Hexanone.
D) Hexanedial.
Answer
560.1k+ views
Hint: We know that alkanes aren't very reactive unless burned and that they will react with reactive chemicals like chlorine and bromine when heated or subjected to UV light to make chlorinated alkane hydrocarbons.
Complete step by step answer:
Despite the reactivity of chlorine we need to do something extra to start the reaction like UV light at temperature or higher temperature. A substitution reaction occurs and a chloro–alkane is made. Therefore cyclohexane in reaction with the chlorine yields chloro cyclohexane.
The ionization of aqueous potassium hydroxide produces hydroxide ions which act as strong nucleophiles. Hence, alkyl chlorides undergo substitution to make alcohol. Alcoholic potassium hydroxide solution gives alkoxide ion which may be a strong base. It abstracts beta hydrogen atoms of alkyl chloride. A molecule of hydrogen chloride is eliminated and an alkene is made. Thus chloro cyclohexane in reaction with alcoholic potassium hydroxide gives cyclohexene.
Ozonolysis refers to the organic reaction in which ozone is used to break the unsaturated bonds. We know that the Ozone may be a very reactive allotrope of oxygen. The reaction of ozone with alkenes and alkynes causes the oxidative cleaving of the alkene or alkyne as a result the carbon-carbon triple bonds are replaced with carbon-oxygen double bonds, giving carbonyl products. Thus, cyclohexene on ozonolysis gives Hexanedial. Hence option D is correct.
The reaction is,
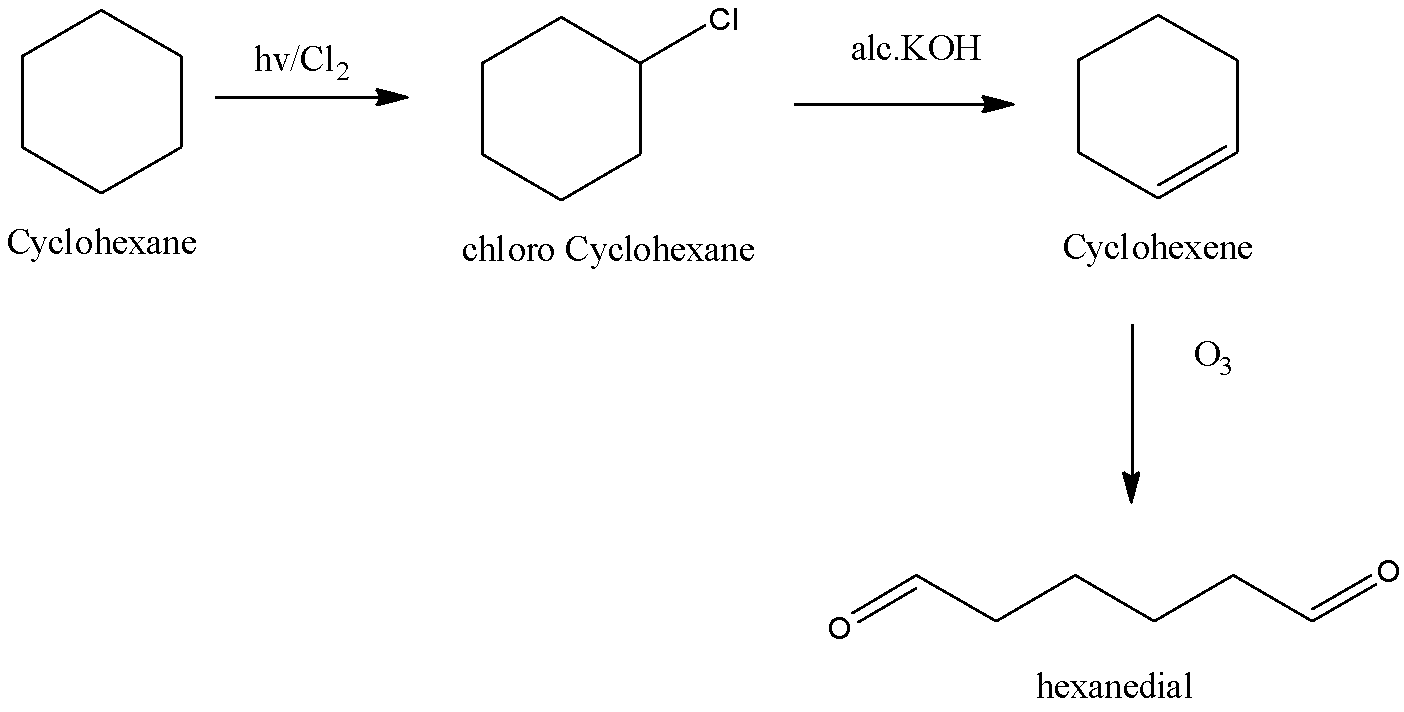
So, the correct answer is Option D.
Note: We have to know that the basicity of hydroxyl ion is far less than the basicity of alkoxide ion as hydroxyl ion is significantly hydrated in solution. Hence, hydroxyl ions cannot abstract the beta atom of alkyl chloride. We have to remember that the cyclohexane is a colourless organic compound with detergent like odour. We have to know that the adipic acid and caprolactam is produced in industries by using cyclohexane.
Complete step by step answer:
Despite the reactivity of chlorine we need to do something extra to start the reaction like UV light at temperature or higher temperature. A substitution reaction occurs and a chloro–alkane is made. Therefore cyclohexane in reaction with the chlorine yields chloro cyclohexane.
The ionization of aqueous potassium hydroxide produces hydroxide ions which act as strong nucleophiles. Hence, alkyl chlorides undergo substitution to make alcohol. Alcoholic potassium hydroxide solution gives alkoxide ion which may be a strong base. It abstracts beta hydrogen atoms of alkyl chloride. A molecule of hydrogen chloride is eliminated and an alkene is made. Thus chloro cyclohexane in reaction with alcoholic potassium hydroxide gives cyclohexene.
Ozonolysis refers to the organic reaction in which ozone is used to break the unsaturated bonds. We know that the Ozone may be a very reactive allotrope of oxygen. The reaction of ozone with alkenes and alkynes causes the oxidative cleaving of the alkene or alkyne as a result the carbon-carbon triple bonds are replaced with carbon-oxygen double bonds, giving carbonyl products. Thus, cyclohexene on ozonolysis gives Hexanedial. Hence option D is correct.
The reaction is,

So, the correct answer is Option D.
Note: We have to know that the basicity of hydroxyl ion is far less than the basicity of alkoxide ion as hydroxyl ion is significantly hydrated in solution. Hence, hydroxyl ions cannot abstract the beta atom of alkyl chloride. We have to remember that the cyclohexane is a colourless organic compound with detergent like odour. We have to know that the adipic acid and caprolactam is produced in industries by using cyclohexane.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




