
In the reaction $Chlorobenzene + Mg\xrightarrow[{ether}]{{dry}}A\xrightarrow{{BIOH}}B$
A. Ethylbenzene
B. Phenol
C. Benzene
D. Phenyl Methyl ether
Answer
582.3k+ views
Hint: For determining, A and B in the given reaction we need to understand the concept of chlorobenzene. Chlorobenzene is an organic compound which is colorless, volatile, water-soluble compound and is used as an intermediate to manufacture other chemical compounds.
Complete step by step answer:By referring to the chemical reaction, we know that the complete reaction takes place in two steps. Firstly, we convert to chlorobenzene to phenyl magnesium chloride. In step-1, the reaction takes place when chlorobenzene is reacted with magnesium in the presence of dry ether then it results in the formation of phenyl magnesium chloride which is also known as Grignard’s reagent.
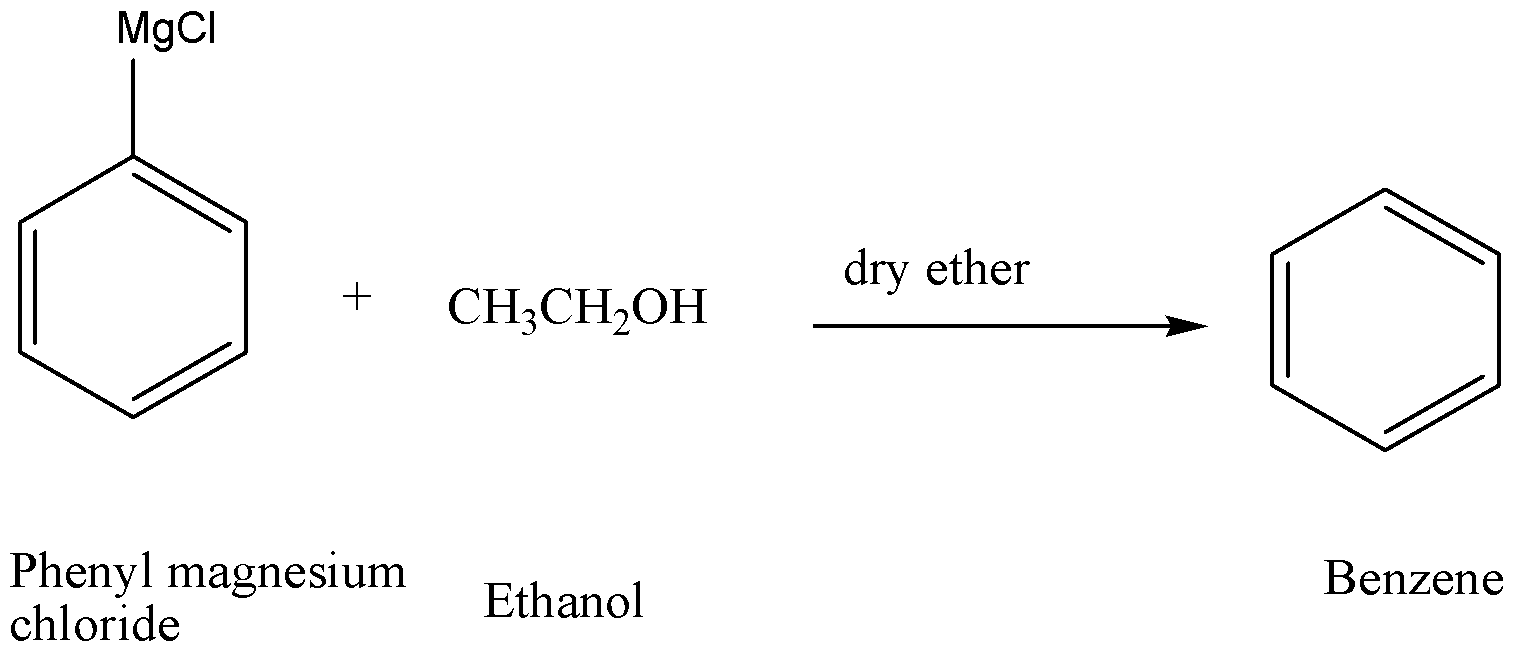
In the step-2, when phenyl magnesium chloride reacts with ethanol then benzene is formed as the resultant of the reaction. Here, ethanol acts as an active hydrogen donor so benzene (option C) is formed.
Now, we will refer to the chemical reactions that take place in both these steps:
Step-1
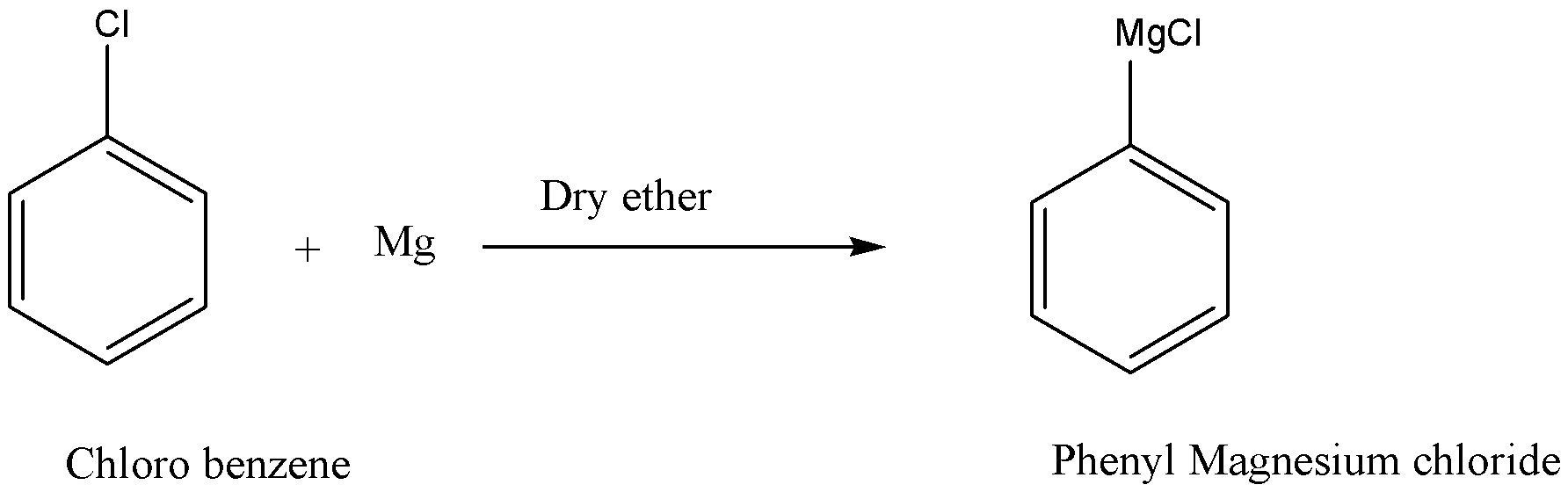
Step-2
We can write the complete chemical reaction as,
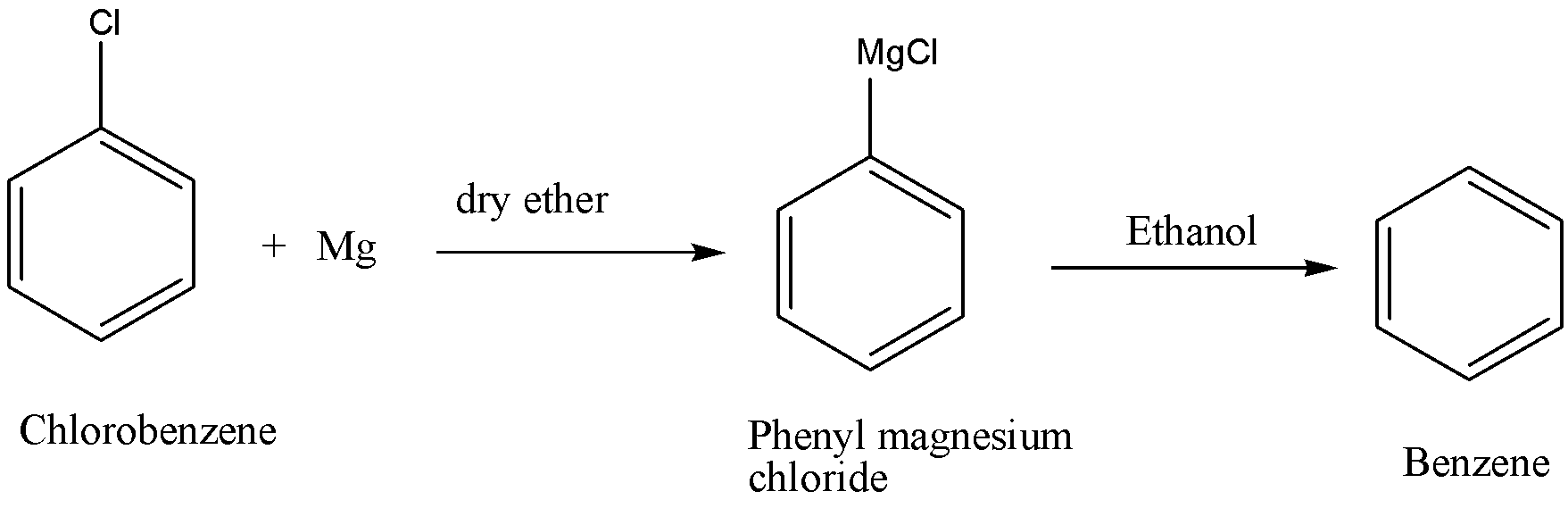
The benzene obtained in the above chemical reaction possesses chemical formula ${C_6}{H_6}$. It is an organic compound which is composed of six Carbon atoms that are joined in a planar ring with one hydrogen atom attached to each Carbon atom. As the chemical compound contains only carbon and hydrogen atoms so we can say that benzene is a hydrocarbon.
Note:After solving this reaction, we need to remember that industries use benzene to make chemicals which are used to make plastics, resins, and nylon and synthetic fibers. Also, it is used to make lubricants, rubbers, dyes, detergents, drugs, and pesticides.
Complete step by step answer:By referring to the chemical reaction, we know that the complete reaction takes place in two steps. Firstly, we convert to chlorobenzene to phenyl magnesium chloride. In step-1, the reaction takes place when chlorobenzene is reacted with magnesium in the presence of dry ether then it results in the formation of phenyl magnesium chloride which is also known as Grignard’s reagent.
In the step-2, when phenyl magnesium chloride reacts with ethanol then benzene is formed as the resultant of the reaction. Here, ethanol acts as an active hydrogen donor so benzene (option C) is formed.
Now, we will refer to the chemical reactions that take place in both these steps:
Step-1

Step-2

We can write the complete chemical reaction as,

The benzene obtained in the above chemical reaction possesses chemical formula ${C_6}{H_6}$. It is an organic compound which is composed of six Carbon atoms that are joined in a planar ring with one hydrogen atom attached to each Carbon atom. As the chemical compound contains only carbon and hydrogen atoms so we can say that benzene is a hydrocarbon.
Note:After solving this reaction, we need to remember that industries use benzene to make chemicals which are used to make plastics, resins, and nylon and synthetic fibers. Also, it is used to make lubricants, rubbers, dyes, detergents, drugs, and pesticides.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




