
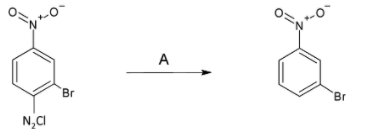
In the reaction A is:
A.$KI/\Delta $
B.$CuCl/HCl$
C.${{H}_{3}}P{{O}_{2}}$
D.${{H}_{2}}O/\Delta $

Answer
573.3k+ views
Hint:In the question we are given with two organic structures and we need to answer about the reagent used. We will check the use of the reagents in the given options and find the answer.
Complete step by step solution:
In the first compound there is a diazonium group present with the compound. On the products side we see that the diazonium group is not present and all is the same as the reactant.
The first option is $KI/\Delta $ which is generally used for substitution of a substituent by iodine. In reaction with the compound $KI/\Delta $ will change the diazonium group by iodine.
In the second option we have $CuCl/HCl$. This is a specific reagent which is used to convert the diazonium group to chlorine group. But in the required product we are not given with any chlorine substituent. So this is not the answer.
In the third option we have ${{H}_{3}}P{{O}_{2}}$. In the question we just want to convert the diazonium group to hydrogen by reduction. So if we use this particular reagent and then hydrolyse the product obtained we get the required product. From this we get to know that this is the answer. But we will check the next option.
In the fourth option we have ${{H}_{2}}O/\Delta $. On reacting diazonium with water we get phenol. So this will not be our answer.
So from all the above analysis we get the answer as option C.
Note:
It is very important to know the uses of various reagents. If it is known then this type of question becomes very easy. It should be noted that in organic chemistry uses of reagents should be known along with this the names of reducing or oxidizing agents should be known.
Complete step by step solution:
In the first compound there is a diazonium group present with the compound. On the products side we see that the diazonium group is not present and all is the same as the reactant.
The first option is $KI/\Delta $ which is generally used for substitution of a substituent by iodine. In reaction with the compound $KI/\Delta $ will change the diazonium group by iodine.
In the second option we have $CuCl/HCl$. This is a specific reagent which is used to convert the diazonium group to chlorine group. But in the required product we are not given with any chlorine substituent. So this is not the answer.
In the third option we have ${{H}_{3}}P{{O}_{2}}$. In the question we just want to convert the diazonium group to hydrogen by reduction. So if we use this particular reagent and then hydrolyse the product obtained we get the required product. From this we get to know that this is the answer. But we will check the next option.
In the fourth option we have ${{H}_{2}}O/\Delta $. On reacting diazonium with water we get phenol. So this will not be our answer.
So from all the above analysis we get the answer as option C.
Note:
It is very important to know the uses of various reagents. If it is known then this type of question becomes very easy. It should be noted that in organic chemistry uses of reagents should be known along with this the names of reducing or oxidizing agents should be known.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




