
In the given reaction, the final compound (L) is:
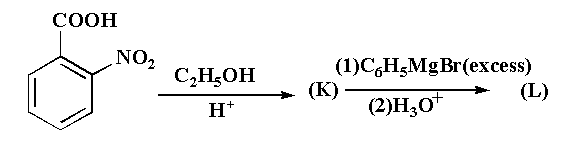

Answer
559.8k+ views
Hint: The given reaction proceeds in three steps. In the first step carboxylic acid is converted to ester by substitution reaction. In the second step Grignard reagents on reacting with ester forms and intermediate which further on acid hydrolysis gives alcohol.
Complete step by step answer:
This reaction is taking place usually in three step, in first step 2-nitrobenzoic acid is reacted with ethanol in acidic medium to second step 2-nitrobenzoate is reacted with excess of Grignard reagent to form an intermediate. In the third step the intermediate is reacted with hydronium ion gives the final product (2-nitrophenyl)diphenylmethanol as a final product.
The reaction is shown below.
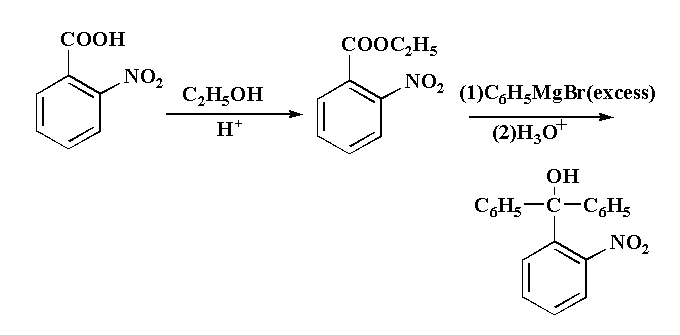
The mechanism of the reaction is that, in the given reaction, first the lone pairs present in the oxygen atom of ethanol attack the hydrogen ion from the acidic medium to form ${C_2}{H_5}{O^ + }{H_2}$, then removal of water takes place which result in the formation of $C{H_3} - CH_2^ +$. The lone pair present in the oxygen of the hydroxyl group attached to the carbon of the carboxylic group of 2-nitrobenzoic acid attack the carbocation $C{H_3} - CH_2^ +$ to form 2-nitrobenzoate which is an ester. The compound 2-nitrobenzoate is then reacted with benzyl magnesium bromide (Grignard reagent) where the ${C_6}H_5^ -$ attack the carbonyl carbon and gets attached to it by removing ${C_2}{H_5}COMgBr$ and then the negative oxygen ion attack the hydronium ion to form the final product (2 nitrophenyl)diphenylmethanol.
The final product L is (2 nitrophenyl)diphenylmethanol.
Note:
In the final product, you can see that two phenyl groups are attached to the carbon, this is because phenyl magnesium bromide (Grignard reagent) is present in excess quantity so it is added twice in the reaction.
Complete step by step answer:
This reaction is taking place usually in three step, in first step 2-nitrobenzoic acid is reacted with ethanol in acidic medium to second step 2-nitrobenzoate is reacted with excess of Grignard reagent to form an intermediate. In the third step the intermediate is reacted with hydronium ion gives the final product (2-nitrophenyl)diphenylmethanol as a final product.
The reaction is shown below.

The mechanism of the reaction is that, in the given reaction, first the lone pairs present in the oxygen atom of ethanol attack the hydrogen ion from the acidic medium to form ${C_2}{H_5}{O^ + }{H_2}$, then removal of water takes place which result in the formation of $C{H_3} - CH_2^ +$. The lone pair present in the oxygen of the hydroxyl group attached to the carbon of the carboxylic group of 2-nitrobenzoic acid attack the carbocation $C{H_3} - CH_2^ +$ to form 2-nitrobenzoate which is an ester. The compound 2-nitrobenzoate is then reacted with benzyl magnesium bromide (Grignard reagent) where the ${C_6}H_5^ -$ attack the carbonyl carbon and gets attached to it by removing ${C_2}{H_5}COMgBr$ and then the negative oxygen ion attack the hydronium ion to form the final product (2 nitrophenyl)diphenylmethanol.
The final product L is (2 nitrophenyl)diphenylmethanol.
Note:
In the final product, you can see that two phenyl groups are attached to the carbon, this is because phenyl magnesium bromide (Grignard reagent) is present in excess quantity so it is added twice in the reaction.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




