
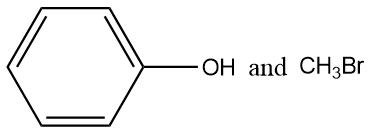
In the given reaction, products are:
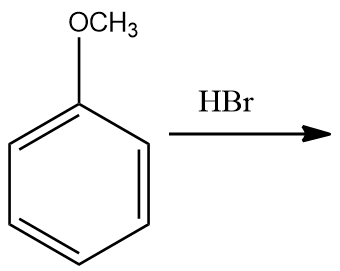
(A)
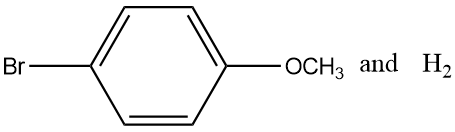
(B)
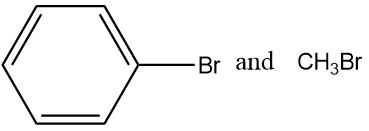
(C)
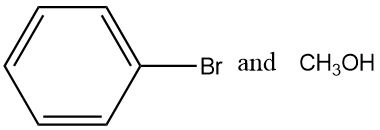
(D)





Answer
579.6k+ views
Hint: Ethers are very less reactive for substitution because alkoxide ions are a very poor leaving group. Under very strong conditions, they undergo protonation and become a leaving group and undergo substitution.
Complete step by step answer:
On reaction of Anisole and HBr, substitution reaction takes place. The products of the reaction are Phenol and methyl bromide.
$S{{N}_{1}}$ reaction occurs when stable carbocation intermediate is formed.
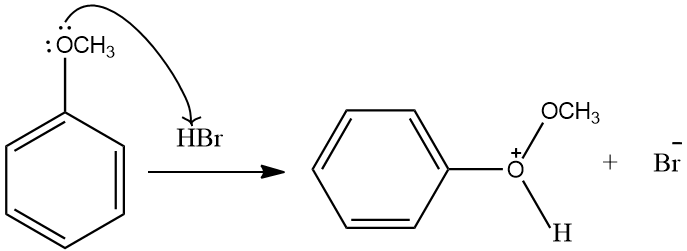
In the following reaction, lone pairs on oxygen , attacks on the proton of the HBr. The Methoxy group on the anisole gets protonated. After protonation it forms methyl phenyl oxonium ion. Then the methoxy group leaves the compound thereby giving the group. The methoxy group now reacts with the Bromide ion, to give methyl bromide.
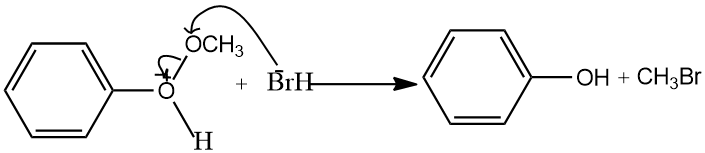
Cleavage of methoxy group from the oxonium ion takes place, because oxygen atom attached to the benzene ring have a partial double bond character due to the resonance, therefore, oxygen benzene bond was stronger than oxygen methoxy bond, therefore, cleavage of methoxy group from the oxonium ion takes place.
So, the correct answer is “Option D”.
Note: The $S{{N}_{1}}$ reaction is a substitution nucleophilic unimolecular reaction. It is a two-step reaction. In the first step, The carbon-halogen bond breaks heterolytically with the halogen retaining the previously shared pair of electrons. In the second step, the nucleophile reacts rapidly with the carbocation that was formed in the first step.This reaction is carried out in polar protic solvents such as water, alcohol, acetic acid etc
Complete step by step answer:
On reaction of Anisole and HBr, substitution reaction takes place. The products of the reaction are Phenol and methyl bromide.
$S{{N}_{1}}$ reaction occurs when stable carbocation intermediate is formed.
In the following reaction, lone pairs on oxygen , attacks on the proton of the HBr. The Methoxy group on the anisole gets protonated. After protonation it forms methyl phenyl oxonium ion. Then the methoxy group leaves the compound thereby giving the group. The methoxy group now reacts with the Bromide ion, to give methyl bromide.


Cleavage of methoxy group from the oxonium ion takes place, because oxygen atom attached to the benzene ring have a partial double bond character due to the resonance, therefore, oxygen benzene bond was stronger than oxygen methoxy bond, therefore, cleavage of methoxy group from the oxonium ion takes place.
So, the correct answer is “Option D”.
Note: The $S{{N}_{1}}$ reaction is a substitution nucleophilic unimolecular reaction. It is a two-step reaction. In the first step, The carbon-halogen bond breaks heterolytically with the halogen retaining the previously shared pair of electrons. In the second step, the nucleophile reacts rapidly with the carbocation that was formed in the first step.This reaction is carried out in polar protic solvents such as water, alcohol, acetic acid etc
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




