
In the following sequence of reaction, $C{H_3}Br\xrightarrow{{KCN}}A\xrightarrow{{{H_3}{O^ + }}}B\xrightarrow{{LiAl{H_4}}}C$
$(a)$ Ethyl alcohol
$(b)$ Acetaldehyde
$(c)$ Acetone
$(d)$ Methane
Answer
500.4k+ views
Hint: In these types of questions we need to know what the reactant is undergoing with what type of catalyst and need to understand the mechanism during the formation. Step by step we need to find the compound $A$ and then $B$ and then we get our final product $C$.
Complete answer:
When methyl bromide reacts with potassium cyanide to give cyano methane and potassium bromide. The reaction is ${S_N}2$ reaction. When the nucleophile attacks the carbon with bromine, it forms a transition state which is a combined product which contains both bromine and cyano groups. So, the element $A$ is $C{H_3}CN$.
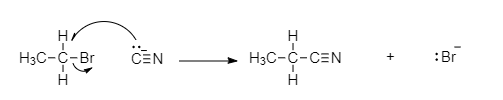
The reaction with cyano methane with hydronium ion is an acid catalyzed hydrolysis, once water has reacted with the nitrile carbon, a proton transfer and resonance occur to produce a protonated amide. Water acts as a weak base, deprotonating the carbonyl to form an amide and regenerating the hydronium catalyst. Further hydrolysis converts the amide to the carboxylic acid. So, the element $B$ is $C{H_3}COOH$.
$C{H_3}CN\xrightarrow{{{H_3}{O^ + }}}C{H_3}COOH$
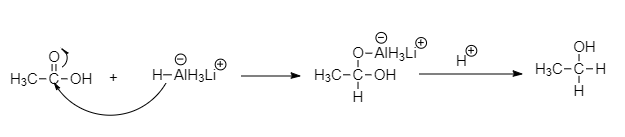
Lithium aluminum hydride is a strong reducing agent, reduction of carboxylic acid or acetic acid to form alcohol. The nucleophile $H$ from the hydride reagent adds to the electrophilic carbon in the polar carboxyl group of acetic acid. So, the element $C$ is ethanol.
Thus, the correct answer is option A.
Note:
Aldehyde gets converted to primary alcohol, similarly ketone on reaction with lithium aluminum hydride gets converted to secondary alcohol. Ester gets converted to two different alcohols depending upon the alkyl group attached. Carboxylic acid gets converted to alcohol. Similarly amide gets converted to amine.
Complete answer:
When methyl bromide reacts with potassium cyanide to give cyano methane and potassium bromide. The reaction is ${S_N}2$ reaction. When the nucleophile attacks the carbon with bromine, it forms a transition state which is a combined product which contains both bromine and cyano groups. So, the element $A$ is $C{H_3}CN$.

The reaction with cyano methane with hydronium ion is an acid catalyzed hydrolysis, once water has reacted with the nitrile carbon, a proton transfer and resonance occur to produce a protonated amide. Water acts as a weak base, deprotonating the carbonyl to form an amide and regenerating the hydronium catalyst. Further hydrolysis converts the amide to the carboxylic acid. So, the element $B$ is $C{H_3}COOH$.
$C{H_3}CN\xrightarrow{{{H_3}{O^ + }}}C{H_3}COOH$
Lithium aluminum hydride is a strong reducing agent, reduction of carboxylic acid or acetic acid to form alcohol. The nucleophile $H$ from the hydride reagent adds to the electrophilic carbon in the polar carboxyl group of acetic acid. So, the element $C$ is ethanol.

Thus, the correct answer is option A.
Note:
Aldehyde gets converted to primary alcohol, similarly ketone on reaction with lithium aluminum hydride gets converted to secondary alcohol. Ester gets converted to two different alcohols depending upon the alkyl group attached. Carboxylic acid gets converted to alcohol. Similarly amide gets converted to amine.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




