
In the following reaction,
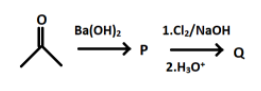
Q is:
(A).
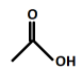
(B).
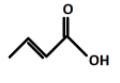
(C).

(D).
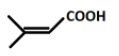





Answer
570k+ views
Hint: The reaction of acetone with $Ba{{(OH)}_{2}}$ refers to the condensation reaction between the two reactants molecules. In the reaction the new carbon- carbon bonds formed after the combination of the both reactants molecules. There will be elimination of a simple molecule and then the product formed will react with chlorine and sodium hydroxide to form the product.
Complete step by step answer:
The initial structure is of acetone, having the molecular formula, $C{{H}_{3}}COC{{H}_{3}}$ which is a common name for propanone. Another name for acetone is dimethyl ketone. It is used as a solvent in many reactions and it is volatile which means that if it is kept open then it will evaporate.
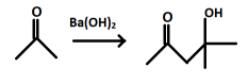
In the first step acetone reacts with barium hydroxide and forms a product P. When $Ba{{(OH)}_{2}}$ is added to acetone some of the molecules of acetone will undergoes enolization and production of enol will take place. The product formed after the reaction is a $\beta -$ hydroxy ketone and has 2 functional groups.
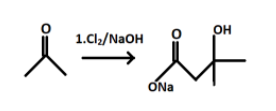
The product P formed will react with $C{{l}_{2}}/NaOH$ and leads to formation of sodium salt of the compound P.
And after hydrolysis the final product formed i.e. Q will be:
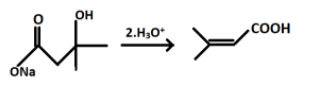
Hence, the correct answer is option (d).
Note: Carbon of the carbonyl group is not reactive towards the electrophilic but oxygen of the carbonyl group will be reactive towards the electrophile. The condensation reaction can take place between two different types of the carbonyl compounds. If the reaction is between two different ketones and aldehydes or ketones then these reactions are known as cross condensation reactions.
Complete step by step answer:
The initial structure is of acetone, having the molecular formula, $C{{H}_{3}}COC{{H}_{3}}$ which is a common name for propanone. Another name for acetone is dimethyl ketone. It is used as a solvent in many reactions and it is volatile which means that if it is kept open then it will evaporate.
In the first step acetone reacts with barium hydroxide and forms a product P. When $Ba{{(OH)}_{2}}$ is added to acetone some of the molecules of acetone will undergoes enolization and production of enol will take place. The product formed after the reaction is a $\beta -$ hydroxy ketone and has 2 functional groups.

The product P formed will react with $C{{l}_{2}}/NaOH$ and leads to formation of sodium salt of the compound P.

And after hydrolysis the final product formed i.e. Q will be:

Hence, the correct answer is option (d).
Note: Carbon of the carbonyl group is not reactive towards the electrophilic but oxygen of the carbonyl group will be reactive towards the electrophile. The condensation reaction can take place between two different types of the carbonyl compounds. If the reaction is between two different ketones and aldehydes or ketones then these reactions are known as cross condensation reactions.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




