
In the following reaction, addition is called
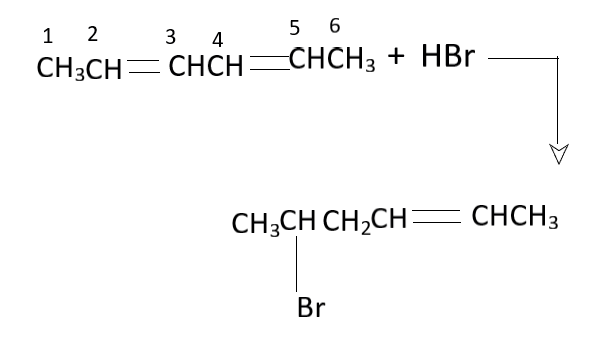
A. $2,3$ addition
B. $1,2$ addition
C. $1,4$ addition-addition
D. $4,5$ addition

Answer
564.9k+ views
Hint:As the bonds in alkene residue breaks there is the formation of a partial positive charge $\partial \left( + \right)$ and the partial negative charge $\partial \left( - \right)$. The electrophilic or nucleophilic attack of the reacting elements with the organic molecule takes place based on this charge.
Complete step by step answer:
Due to the action of the \[HBr\] on the residue with the double bonded structure, the $\pi $ bond breaks and there is the formation of a partial positive charge on one of the carbon, while there is the formation of a partial negative charge on the other carbon. The electrophile is attracted to the partial negative charge while the nucleophile is attracted to the partial positive charge. The \[HBr\] is the reactant which is converted to ${H^ + }$ and $B{r^ - }$ ions. The ${2^o}$ carbocation is the one which has stability while the terminal carbanion has is the structure. This is why the partial positive charge is formed in the carbon number $2$ while the partial negative charge is formed on the carbon number $1$ of the given molecule. The nucleophile $B{r^ - }$ is associated with the partial positive charge, which is why the linkage of bromide occurs at the carbon number $2$. The hydrogen ${H^{{ + ^{}}}}$ is the electrophile which bonds at the carbon with the partially negative charge. Therefore, a simple addition of the components of \[HBr\] takes place and this is known as $1,2$ addition. This is because the addition of the ions takes place in the carbon numbers $1$ and $2$ of the organic molecule.
So the correct answer for the given question is option B.
Note:
The addition reaction is the process in which one of the reactant components is added to the other molecule. The addition of specific components of this reactant is possible for the formation of a new molecule as the product with this association.
Complete step by step answer:
Due to the action of the \[HBr\] on the residue with the double bonded structure, the $\pi $ bond breaks and there is the formation of a partial positive charge on one of the carbon, while there is the formation of a partial negative charge on the other carbon. The electrophile is attracted to the partial negative charge while the nucleophile is attracted to the partial positive charge. The \[HBr\] is the reactant which is converted to ${H^ + }$ and $B{r^ - }$ ions. The ${2^o}$ carbocation is the one which has stability while the terminal carbanion has is the structure. This is why the partial positive charge is formed in the carbon number $2$ while the partial negative charge is formed on the carbon number $1$ of the given molecule. The nucleophile $B{r^ - }$ is associated with the partial positive charge, which is why the linkage of bromide occurs at the carbon number $2$. The hydrogen ${H^{{ + ^{}}}}$ is the electrophile which bonds at the carbon with the partially negative charge. Therefore, a simple addition of the components of \[HBr\] takes place and this is known as $1,2$ addition. This is because the addition of the ions takes place in the carbon numbers $1$ and $2$ of the organic molecule.
So the correct answer for the given question is option B.
Note:
The addition reaction is the process in which one of the reactant components is added to the other molecule. The addition of specific components of this reactant is possible for the formation of a new molecule as the product with this association.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

Give 10 examples of unisexual and bisexual flowers




