
In the above reaction ‘X’ stands for:
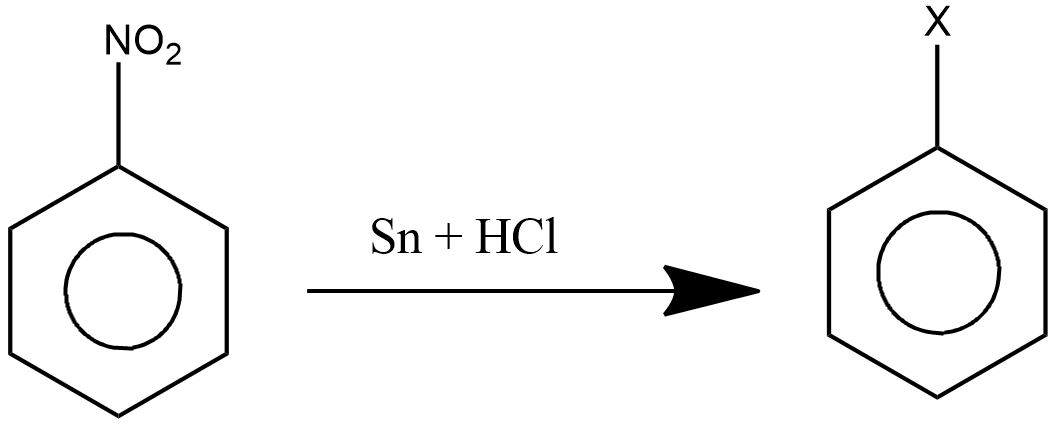
A.\[N{H_2}\]
B.\[SnC{l_2}\]
C.\[Cl\]
D.\[N{H_4}^ + C{l^ - }\]

Answer
515.7k+ views
Hint: When nitrobenzene is subjected to reaction with tin ( \[Sn\] ) and hydrochloric acid ( \[HCl\] ), it undergoes reduction. And the final product we get is aniline, which is the reduced product of nitrobenzene.
Complete answer:
Here we have been given a reaction in which nitrobenzene is subjected to a reaction with tin ( \[Sn\] ) and hydrochloric acid ( \[HCl\] ). The product formed on the right hand side will be aniline.
Aniline is shown below
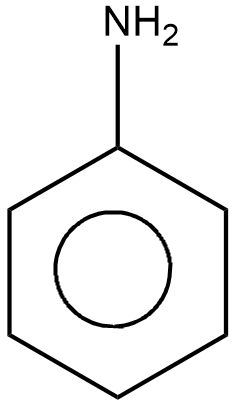
Thus, the X in the above reaction stands for \[N{H_2}\] . Thus, the correct option is A, \[N{H_2}\] .
Now, when nitrobenzene is subjected towards reaction with tin ( \[Sn\] ) and hydrochloric acid ( \[HCl\] ), it undergoes reduction and forms aniline. This reaction is called reduction of nitrobenzene as nitrobenzene gets reduced to aniline. The oxygen in the nitrobenzene is replaced with hydrogen forming ammonia group
In this reaction tin ( \[Sn\] ) and hydrochloric acid ( \[HCl\] ) acts as reducing agent, due to which \[N{H_2}\] replaces \[N{O_2}\] group from the benzene ring which makes it aniline. This reaction can also be performed with iron ( \[Fe\] ) and hydrochloric acid ( \[HCl\] ), they also act as reducing agents in the reaction forming aniline from nitrobenzene.
When nitrobenzene is subjected to reduction with the help of tin ( \[Sn\] ) and hydrochloric acid ( \[HCl\] ), water will be formed as a by-product. Also, the hydrochloric acid in this reaction is concentrated.
And hence the correct option is A.
Note:
The reduction of nitrobenzene to aniline can also be done with the help of iron ( \[Fe\] ) and hydrochloric acid ( \[HCl\] ), and not just tin ( \[Sn\] ) and hydrochloric acid ( \[HCl\] ). Thus, any of the methods can be used for the reduction of nitrobenzene as per the requirements of the maker.
Complete answer:
Here we have been given a reaction in which nitrobenzene is subjected to a reaction with tin ( \[Sn\] ) and hydrochloric acid ( \[HCl\] ). The product formed on the right hand side will be aniline.
Aniline is shown below

Thus, the X in the above reaction stands for \[N{H_2}\] . Thus, the correct option is A, \[N{H_2}\] .
Now, when nitrobenzene is subjected towards reaction with tin ( \[Sn\] ) and hydrochloric acid ( \[HCl\] ), it undergoes reduction and forms aniline. This reaction is called reduction of nitrobenzene as nitrobenzene gets reduced to aniline. The oxygen in the nitrobenzene is replaced with hydrogen forming ammonia group
In this reaction tin ( \[Sn\] ) and hydrochloric acid ( \[HCl\] ) acts as reducing agent, due to which \[N{H_2}\] replaces \[N{O_2}\] group from the benzene ring which makes it aniline. This reaction can also be performed with iron ( \[Fe\] ) and hydrochloric acid ( \[HCl\] ), they also act as reducing agents in the reaction forming aniline from nitrobenzene.
When nitrobenzene is subjected to reduction with the help of tin ( \[Sn\] ) and hydrochloric acid ( \[HCl\] ), water will be formed as a by-product. Also, the hydrochloric acid in this reaction is concentrated.
And hence the correct option is A.
Note:
The reduction of nitrobenzene to aniline can also be done with the help of iron ( \[Fe\] ) and hydrochloric acid ( \[HCl\] ), and not just tin ( \[Sn\] ) and hydrochloric acid ( \[HCl\] ). Thus, any of the methods can be used for the reduction of nitrobenzene as per the requirements of the maker.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




