
In $SO_3^{2 - }$ :
A.$d\pi - p\pi $ bond between $S$ and $O$ is delocalized.
B.Bonds between $S$ and $O$ are equivalent.
C.There is $s{p^3}$ hybridized sulphur atom
D.All of the facts given above are true.
Answer
569.7k+ views
Hint:
$SO_3^{2 - }$ known as sulphite is a sulphur oxoanion. They are naturally occurring substances present in some foods. The oxidation state of sulphur in sulphite is $ + 4$ . Sulphite also shows resonance effect.
Complete step by step answer:
The Lewis structure of $SO_3^{2 - }$ is as follows:
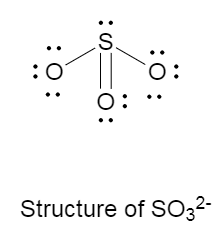
There is one double bond, two single bonds and one lone pair on the central atom that is sulphur.
The molecular structure of sulfite anion is trigonal pyramidal, with bond angle of ${109.5^ \circ }$ .
A.$d\pi - p\pi $ bond between $S$ and $O$ is delocalized.
Reason: The d-orbital of sulphur overlaps with the p-orbital of oxygen in order to form $d\pi - p\pi $ bond.
Therefore the above statement is true.
B.the bonds between sulphur and oxygen are same:
Reason: The bonds between sulphur and oxygen are the same because of the resonance effect.
Resonance effect is the shifting of double bonds.
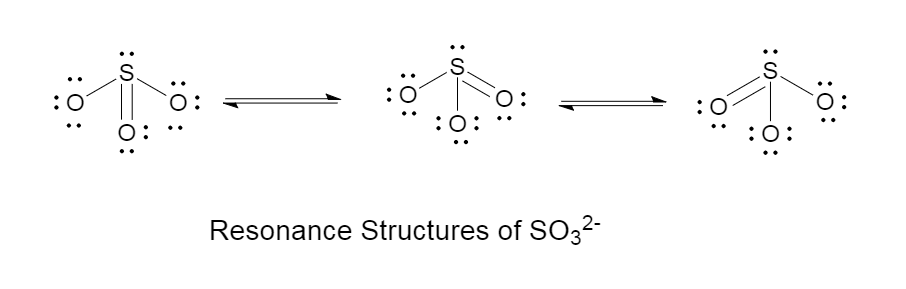
The resonance effect of sulphite is given as follows in the diagram:
C.There is $s{p^3}$ hybridized sulphur atom.
In order to check the hybridization of atom we will use the following formula:
Total number of attached atoms $ + $ number of lone pairs .
For $sp$ hybridization: Total number of attached atoms $ + $ number of lone pair $ = 2$
For $s{p^2}$ hybridization: Total number of attached atoms $ + $ number of lone pair $ = 3$
For $s{p^3}$ hybridization: Total number of attached atoms $ + $ number of lone pair $ = 4$
Now we will calculate the hybridization sulphur in $SO_3^{2 - }$ :
Total number of attached atoms present in sulphur is $3$ .
And the number of lone pairs is $1$.
$ \Rightarrow $ Total number of attached atoms to sulphur $ + $ number of lone pair on sulphur
Substituting the values we get,
$ = 3 + 1$
$ = 4$
Therefore the hybridization of sulphur in sulphite is $s{p^3}$ .
Hence the above statement is true.
Therefore all the statements about sulphite are true.
So the correct answer is option D i.e all of the above.
So the appropriate option would be option C.
Note:Due to the presence of lone pairs of electrons on sulphur, they try to push the bonds thus causing the structure to be trigonal pyramidal. Sulfite ion is the conjugate base of bisulphite.
$SO_3^{2 - }$ known as sulphite is a sulphur oxoanion. They are naturally occurring substances present in some foods. The oxidation state of sulphur in sulphite is $ + 4$ . Sulphite also shows resonance effect.
Complete step by step answer:
The Lewis structure of $SO_3^{2 - }$ is as follows:

There is one double bond, two single bonds and one lone pair on the central atom that is sulphur.
The molecular structure of sulfite anion is trigonal pyramidal, with bond angle of ${109.5^ \circ }$ .
A.$d\pi - p\pi $ bond between $S$ and $O$ is delocalized.
Reason: The d-orbital of sulphur overlaps with the p-orbital of oxygen in order to form $d\pi - p\pi $ bond.
Therefore the above statement is true.
B.the bonds between sulphur and oxygen are same:
Reason: The bonds between sulphur and oxygen are the same because of the resonance effect.
Resonance effect is the shifting of double bonds.
The resonance effect of sulphite is given as follows in the diagram:

C.There is $s{p^3}$ hybridized sulphur atom.
In order to check the hybridization of atom we will use the following formula:
Total number of attached atoms $ + $ number of lone pairs .
For $sp$ hybridization: Total number of attached atoms $ + $ number of lone pair $ = 2$
For $s{p^2}$ hybridization: Total number of attached atoms $ + $ number of lone pair $ = 3$
For $s{p^3}$ hybridization: Total number of attached atoms $ + $ number of lone pair $ = 4$
Now we will calculate the hybridization sulphur in $SO_3^{2 - }$ :
Total number of attached atoms present in sulphur is $3$ .
And the number of lone pairs is $1$.
$ \Rightarrow $ Total number of attached atoms to sulphur $ + $ number of lone pair on sulphur
Substituting the values we get,
$ = 3 + 1$
$ = 4$
Therefore the hybridization of sulphur in sulphite is $s{p^3}$ .
Hence the above statement is true.
Therefore all the statements about sulphite are true.
So the correct answer is option D i.e all of the above.
So the appropriate option would be option C.
Note:Due to the presence of lone pairs of electrons on sulphur, they try to push the bonds thus causing the structure to be trigonal pyramidal. Sulfite ion is the conjugate base of bisulphite.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




