
In Nelson’s cells, NaOH are formed at _____ (cathode/anode) electrode.
Answer
589.5k+ views
Hint: The device which converts electrical energy into chemical energy is known as an electrolytic cell. It consists of a positively charged anode and a negatively charged cathode. Electrical energy brings out a chemical reaction with the help of external energy sources.
Complete step by step solution:
Nelson cell is an example of an electrolytic cell where the redox reaction undergoes an application of electrical energy.
In the nelson cell, sodium hydroxide production from a brine solution is an electrolytic process.
Construction and working of Nelson cell process:
The principle of this process is a porous diaphragm. It consists of a perforated steel tube lined inside with asbestos. It is a suspended steel tank.
Cathode: the tube acts as a cathode
Anode: a graphic rod dipped in sodium chloride solution serves as an anode
Electrolyte: 10% sodium chloride solution (brine solution)
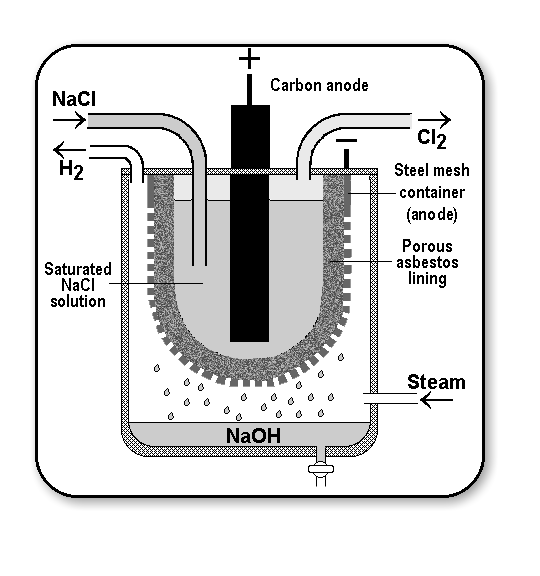
Process: due to this process is an electrolytic, electric energy should be supplied from an outer energy source. On passing electric current, chlorine is liberated at the anode and let out through the outlet. Sodium ions penetrate through the asbestos and reach the cathode when hydrogen and hydroxide ions are formed by the reduction of water. Sodium ions combine with hydroxyl ions to form NaOH which is collected in the outer tank while hydrogen is drawn off through the outlet.
Reactions at the anode: $2C{{l}^{-}}\to C{{l}_{2}}+2{{e}^{-}}$
Reactions at the cathode: $\begin{align}
& 2{{H}_{2}}O+2{{e}^{-}}\rightleftharpoons {{H}_{2}}+2O{{H}^{-}} \\
& N{{a}^{+}}+O{{H}^{-}}\rightleftharpoons NaOH \\
\end{align}$
From the above reactions, NaOH is formed at the cathode.
Note: In Nelson's cell, during the process, the steam blown keeps the electrolyte warm and helps to keep the perforation clear. The solution containing NaOH and NaCl as an impurity is taken out and evaporated to dryness. The cell gives lower quality of NaOH.
Complete step by step solution:
Nelson cell is an example of an electrolytic cell where the redox reaction undergoes an application of electrical energy.
In the nelson cell, sodium hydroxide production from a brine solution is an electrolytic process.
Construction and working of Nelson cell process:
The principle of this process is a porous diaphragm. It consists of a perforated steel tube lined inside with asbestos. It is a suspended steel tank.
Cathode: the tube acts as a cathode
Anode: a graphic rod dipped in sodium chloride solution serves as an anode
Electrolyte: 10% sodium chloride solution (brine solution)

Process: due to this process is an electrolytic, electric energy should be supplied from an outer energy source. On passing electric current, chlorine is liberated at the anode and let out through the outlet. Sodium ions penetrate through the asbestos and reach the cathode when hydrogen and hydroxide ions are formed by the reduction of water. Sodium ions combine with hydroxyl ions to form NaOH which is collected in the outer tank while hydrogen is drawn off through the outlet.
Reactions at the anode: $2C{{l}^{-}}\to C{{l}_{2}}+2{{e}^{-}}$
Reactions at the cathode: $\begin{align}
& 2{{H}_{2}}O+2{{e}^{-}}\rightleftharpoons {{H}_{2}}+2O{{H}^{-}} \\
& N{{a}^{+}}+O{{H}^{-}}\rightleftharpoons NaOH \\
\end{align}$
From the above reactions, NaOH is formed at the cathode.
Note: In Nelson's cell, during the process, the steam blown keeps the electrolyte warm and helps to keep the perforation clear. The solution containing NaOH and NaCl as an impurity is taken out and evaporated to dryness. The cell gives lower quality of NaOH.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




