
In \[KI{O_3}\], what is the charge on \[K\] atom in Lewis structure of molecule \[?\]
A.\[ + 1\]
B.\[ + 2\]
C.\[ - 1\]
D.\[0\]
Answer
502.2k+ views
Hint: First we have to know that Lewis structures are diagrams that describe the nature of chemical bonding between atoms and its positions in the compound which are connected in the compound. It also defined a base as an electron pair donor and an acid as an electron pair acceptor. It can be drawn if the molecular formula of the compound is known.
Complete answer:
\[KI{O_3}\] is the chemical formula for potassium iodate and it is an oxidising agent. It is an ionic compound that can be prepared by reacting potassium base (\[{K^ + }\]) with iodic acid (\[I{O_3}^ - \]) in the ratio \[1:1\]. Since potassium forms an ionic bond with an iodine molecule.
The Lewis structure of \[KI{O_3}\]is drawn as follows
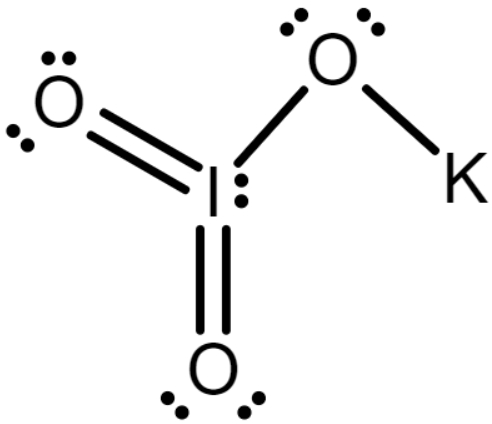
Since the cation potassium has the charge \[ + 1\] and iodic acid has the charge \[ - 1\] in the Lewis structure.
Hence the correct option is (A) \[ + 1\].
Note:
Lewis structure is also called electron dot structure or Lewis dot structure. Also note that while drawing Lewis structures only the valence electrons are considered and the electrons that do not belong to the outermost shell are ignored. When drawing the Lewis structure of the molecule we take the least electronegative atom as the central atom of the molecule and a lone pair can be converted into a bond pair in order to satisfy the octet rule for two atoms.
Complete answer:
\[KI{O_3}\] is the chemical formula for potassium iodate and it is an oxidising agent. It is an ionic compound that can be prepared by reacting potassium base (\[{K^ + }\]) with iodic acid (\[I{O_3}^ - \]) in the ratio \[1:1\]. Since potassium forms an ionic bond with an iodine molecule.
The Lewis structure of \[KI{O_3}\]is drawn as follows

Since the cation potassium has the charge \[ + 1\] and iodic acid has the charge \[ - 1\] in the Lewis structure.
Hence the correct option is (A) \[ + 1\].
Note:
Lewis structure is also called electron dot structure or Lewis dot structure. Also note that while drawing Lewis structures only the valence electrons are considered and the electrons that do not belong to the outermost shell are ignored. When drawing the Lewis structure of the molecule we take the least electronegative atom as the central atom of the molecule and a lone pair can be converted into a bond pair in order to satisfy the octet rule for two atoms.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




