
In FCC structure, the number of tetrahedral voids present along the edges of the FCC unit cell is?
Answer
583.8k+ views
Hint:In the face- centered cubic close packing, each sphere of second layer touches with the three spheres of first layer and thus, they leave a small empty or void space in between, which is known as a tetrahedral void or tetrahedral interstices.
Complete step by step answer:
Tetrahedral void is the vacant space between four touching spheres. Since, one sphere touches three spheres present in the layer below and three spheres present in the above layer, there are two tetrahedral sites associated with one sphere. The structure of a tetrahedral void is shown as:
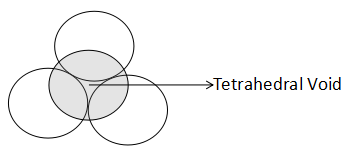
As it is clearly visible, the empty space lying between the two layers is the tetrahedral void. The below layer consists of three spheres and the above layer consists of one layer. The tetrahedral geometry of the interstice can be shown as:
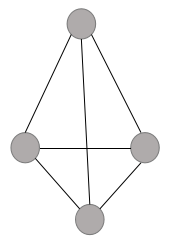
It can be noted here that a tetrahedral site does not mean that the site is tetrahedral in geometry but it means that this site is surrounded by four spheres and by joining the centers of these four spheres, we can form a regular tetrahedron.
Thus, the number of tetrahedral voids present along the edges of the FCC unit cell is zero as all the tetrahedral voids are present along the corners.
Note:
In a FCC structure, one corner sphere and three face spheres form one tetrahedral void. In a FCC structure, two tetrahedral voids are obtained along one cube diagonal. There are a total of four cube diagonals in one unit cell. Thus, overall, there are eight tetrahedral voids in a FCC structure.
Complete step by step answer:
Tetrahedral void is the vacant space between four touching spheres. Since, one sphere touches three spheres present in the layer below and three spheres present in the above layer, there are two tetrahedral sites associated with one sphere. The structure of a tetrahedral void is shown as:

As it is clearly visible, the empty space lying between the two layers is the tetrahedral void. The below layer consists of three spheres and the above layer consists of one layer. The tetrahedral geometry of the interstice can be shown as:

It can be noted here that a tetrahedral site does not mean that the site is tetrahedral in geometry but it means that this site is surrounded by four spheres and by joining the centers of these four spheres, we can form a regular tetrahedron.
Thus, the number of tetrahedral voids present along the edges of the FCC unit cell is zero as all the tetrahedral voids are present along the corners.
Note:
In a FCC structure, one corner sphere and three face spheres form one tetrahedral void. In a FCC structure, two tetrahedral voids are obtained along one cube diagonal. There are a total of four cube diagonals in one unit cell. Thus, overall, there are eight tetrahedral voids in a FCC structure.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




