
In electroplating an article with Nickel, anode is:
(A) An aqueous solution of nickel sulphate
(B) An article to be electroplated
(C) A block of nickel metal
(D) None of these
Answer
552k+ views
Hint: Nickel electroplating is a process of depositing nickel on a metal. The prepared sample is then immersed into an electrolyte solution and is used as the cathode. The nickel anode is dissolved into the electrolyte to form nickel ions. The ions travel through the solution and get deposited on the cathode.
Complete Step By Step Solution
Nickel electroplating is a technique in which a thin layer of nickel is electroplated on a metal. In this process nickel sulfate is used as an electrolyte, an article to be electroplated is placed at cathode and a block of nickel metal is used as anode. So, the correct option is (C) i.e. block of nickel metal is used as anode for the electroplating purpose.
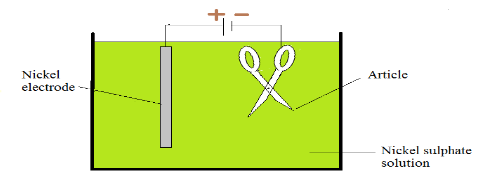
In an electrolytic cell used for electroplating, the object to be plated is used as the cathode. Metal cations are reduced at the cathode and a layer of metal is deposited on the surface of the object. The anode is usually made of the same metal as the one being plated at the cathode. A concentrated solution of ionic compounds of metal being plated is used as the electrolyte. This makes the solution very conductive.
If a nickel electrode is used as the anode, the nickel is oxidized to $ N {{i} ^ {+2}} $ ion, which goes into solution.
Reaction at anode: $N{{i}_{(s)}}\to Ni_{(aq)}^{+2}+2{{e}^{-}}$
At cathode: $Ni {aq} ^ {+2} + 2{{e} ^ {-}}\to Ni_{s} ^ {0} $
The sample gets electroplated with nickel metal.
Note
The metal with which the coating is to be done should be made anode and the article or metal at which coating is to be done should be made cathode. Parts to be plated must be clean and free of dirt, corrosion, and defects before plating can begin. Nickel is very hard and wear resistant. It is used for hard, durable, and corrosion resistant coatings.
Complete Step By Step Solution
Nickel electroplating is a technique in which a thin layer of nickel is electroplated on a metal. In this process nickel sulfate is used as an electrolyte, an article to be electroplated is placed at cathode and a block of nickel metal is used as anode. So, the correct option is (C) i.e. block of nickel metal is used as anode for the electroplating purpose.

In an electrolytic cell used for electroplating, the object to be plated is used as the cathode. Metal cations are reduced at the cathode and a layer of metal is deposited on the surface of the object. The anode is usually made of the same metal as the one being plated at the cathode. A concentrated solution of ionic compounds of metal being plated is used as the electrolyte. This makes the solution very conductive.
If a nickel electrode is used as the anode, the nickel is oxidized to $ N {{i} ^ {+2}} $ ion, which goes into solution.
Reaction at anode: $N{{i}_{(s)}}\to Ni_{(aq)}^{+2}+2{{e}^{-}}$
At cathode: $Ni {aq} ^ {+2} + 2{{e} ^ {-}}\to Ni_{s} ^ {0} $
The sample gets electroplated with nickel metal.
Note
The metal with which the coating is to be done should be made anode and the article or metal at which coating is to be done should be made cathode. Parts to be plated must be clean and free of dirt, corrosion, and defects before plating can begin. Nickel is very hard and wear resistant. It is used for hard, durable, and corrosion resistant coatings.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




