
In dehydrohalogenation of ethyl chloride, which of the following changes occurs?
A.$s{p^2}$carbon converts to $s{p^3}$ carbon
B. $s{p^2}$carbon converts to $sp$ carbon
C. $s{p^3}$carbon converts to $sp$ carbon
D. $s{p^3}$carbon converts to $s{p^2}$ carbon
Answer
594.9k+ views
Hint:
Dehydrohalogenation reaction is a reaction in which a hydrogen halide got removed from a substrate in presence of alcoholic potassium hydroxide.
Complete step by step answer:
First, we have to discuss the structure of ethyl chloride. When one hydrogen of an ethene molecule is replaced by a chlorine atom, ethyl chloride forms.
${\rm{C}}{{\rm{H}}_{\rm{3}}} - {\rm{C}}{{\rm{H}}_2}{\rm{Cl}}$
Now, we understand about the reaction of ethyl chloride with potassium hydroxide. When ethyl chloride reacts with potassium hydroxide both nucleophilic substitution and elimination reaction takes place.
Now, we discuss the elimination reaction of ethyl chloride. In ethyl chloride, $\beta $hydrogens present.
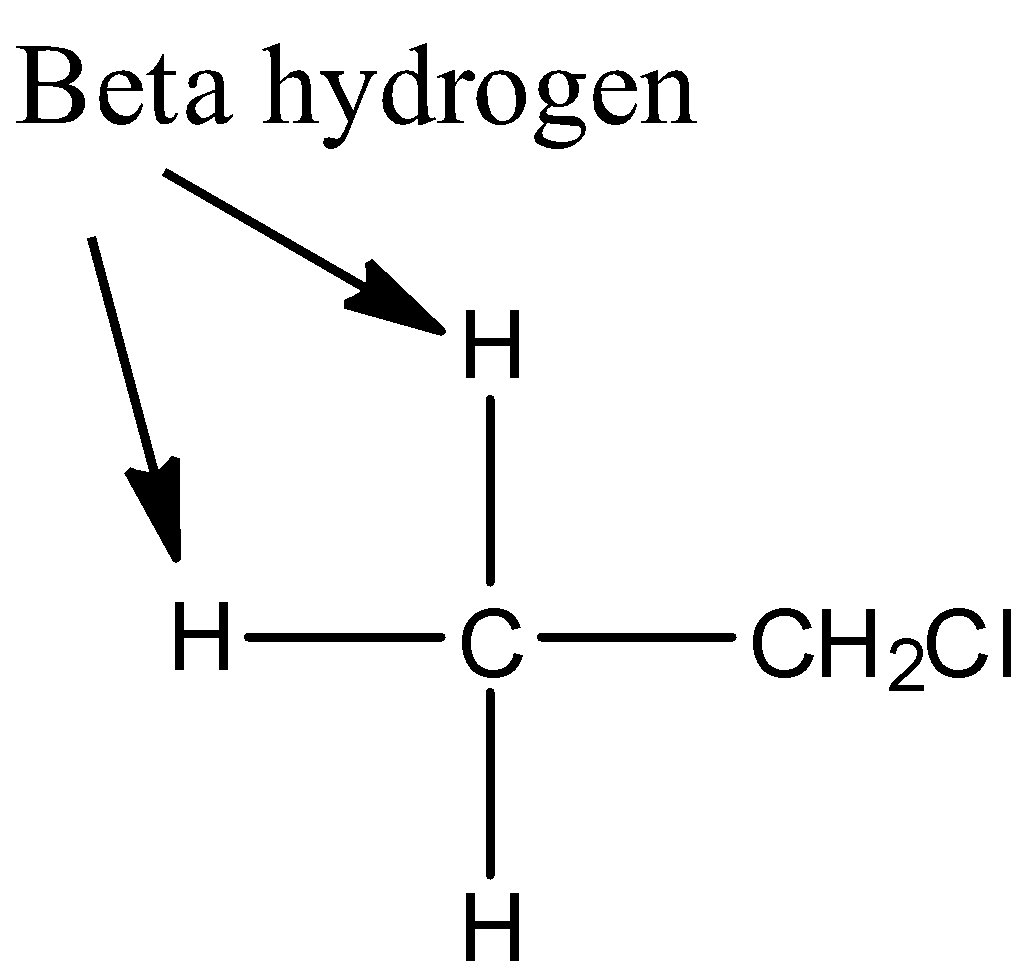
Due to which, it undergoes elimination reaction to produce ethene in presence of strong base like potassium hydroxide.
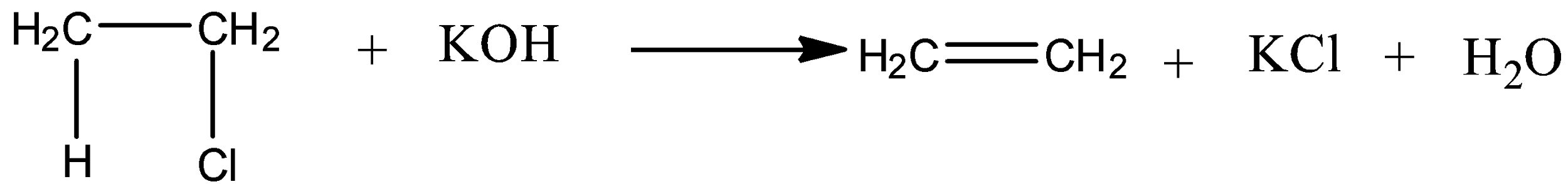
Now, let's discuss the mechanism of dehydrohalogenation reaction of ethyl chloride.
As ethyl chloride is a primary halide, E2 reaction is preferred over E1. E2 reaction is a one step reaction in which a carbon-hydrogen and carbon-halogen bond breaks simultaneously.
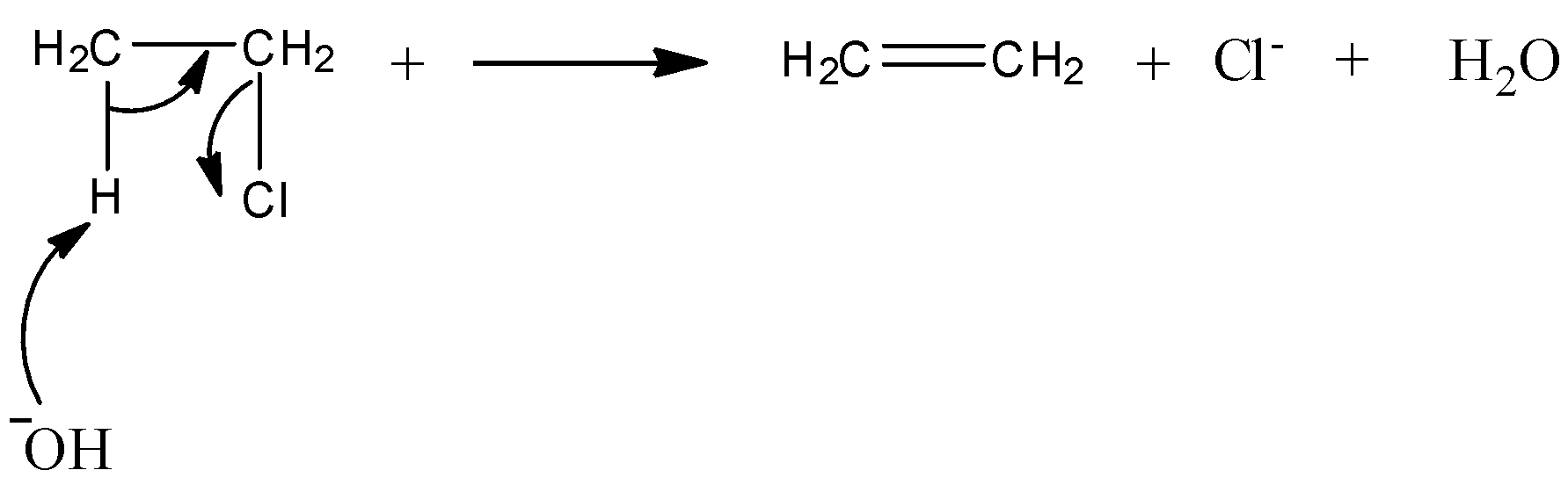
From the above mechanism, we get to know that ethene is formed on reaction of ethyl chloride with potassium hydroxide.
Now, we have to decide the type of carbon in the reactant and product. As the reactant contains only single bonds, so, the type of carbon is ethyl chloride is $s{p^3}$. The product of dehydrohalogenation reaction of ethyl chloride is ethene which contains double bond, so, the type of carbon is ethene is $s{p^2}$. So, the type of carbon in the product is $s{p^2}$, that means, on dehydrohalogenation, $s{p^3}$carbon converts to $s{p^2}$ carbon.
Hence, the correct option is D.
Note:
Ethyl chloride also undergoes nucleophilic substitution on reaction with potassium hydroxide. The nucleophilic substitution reaction results in ethanol and potassium chloride.
${{\rm{C}}_{\rm{2}}}{{\rm{H}}_{\rm{5}}}{\rm{Cl}} + {\rm{KOH}}\left( {aq} \right) \to {{\rm{C}}_{\rm{2}}}{{\rm{H}}_{\rm{5}}}{\rm{OH}} + {\rm{KCl}}$
Dehydrohalogenation reaction is a reaction in which a hydrogen halide got removed from a substrate in presence of alcoholic potassium hydroxide.
Complete step by step answer:
First, we have to discuss the structure of ethyl chloride. When one hydrogen of an ethene molecule is replaced by a chlorine atom, ethyl chloride forms.
${\rm{C}}{{\rm{H}}_{\rm{3}}} - {\rm{C}}{{\rm{H}}_2}{\rm{Cl}}$
Now, we understand about the reaction of ethyl chloride with potassium hydroxide. When ethyl chloride reacts with potassium hydroxide both nucleophilic substitution and elimination reaction takes place.
Now, we discuss the elimination reaction of ethyl chloride. In ethyl chloride, $\beta $hydrogens present.

Due to which, it undergoes elimination reaction to produce ethene in presence of strong base like potassium hydroxide.

Now, let's discuss the mechanism of dehydrohalogenation reaction of ethyl chloride.
As ethyl chloride is a primary halide, E2 reaction is preferred over E1. E2 reaction is a one step reaction in which a carbon-hydrogen and carbon-halogen bond breaks simultaneously.

From the above mechanism, we get to know that ethene is formed on reaction of ethyl chloride with potassium hydroxide.
Now, we have to decide the type of carbon in the reactant and product. As the reactant contains only single bonds, so, the type of carbon is ethyl chloride is $s{p^3}$. The product of dehydrohalogenation reaction of ethyl chloride is ethene which contains double bond, so, the type of carbon is ethene is $s{p^2}$. So, the type of carbon in the product is $s{p^2}$, that means, on dehydrohalogenation, $s{p^3}$carbon converts to $s{p^2}$ carbon.
Hence, the correct option is D.
Note:
Ethyl chloride also undergoes nucleophilic substitution on reaction with potassium hydroxide. The nucleophilic substitution reaction results in ethanol and potassium chloride.
${{\rm{C}}_{\rm{2}}}{{\rm{H}}_{\rm{5}}}{\rm{Cl}} + {\rm{KOH}}\left( {aq} \right) \to {{\rm{C}}_{\rm{2}}}{{\rm{H}}_{\rm{5}}}{\rm{OH}} + {\rm{KCl}}$
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

What organs are located on the left side of your body class 11 biology CBSE

Draw a diagram of nephron and explain its structur class 11 biology CBSE




