
In curing cement plasters water is sprinkled from time to times. This helps in:
A. developing interlocking needle-like crystals of hydrated silicates
B. hydrating sand and gravel mixed with cement
C. converting sand into silicic acid
D. keeping it cool
Answer
578.7k+ views
Hint: For solving this question, we need to understand the concept of cement plasters. We know that cement plaster is a mixture of suitable plaster, sand, Portland cement and water which is normally applied to masonry interiors and exteriors to achieve a smooth surface. Interior surfaces sometimes receive a final layer of gypsum plaster.
Complete step by step answer:
We know that water develops interlocking needles like crystals of hydrated silicates. This reaction involves the hydration of calcium aluminates and calcium silicates which change into their colloidal gels.
At the same time, some calcium hydroxide and aluminum hydroxides are formed as precipitates due to hydrolysis. Calcium hydroxide binds the particles of calcium silicate together while aluminum hydroxide fills the interstices rendering the mass impervious.
Therefore, we can conclude that in curing cement plasters water is sprinkled from time to times to develop interlocking needle-like crystals of hydrated silicates.
Therefore, the option A is correct (developing interlocking needle-like crystals of hydrated silicates).
Note:
For solving this question, we also need to know about the structure of silicates. Silicates are known to show tetrahedral structure in which small open circles represent oxygen atoms and the small closed circle inside represent the silicon atom. We can draw the structure of silicate as given below,
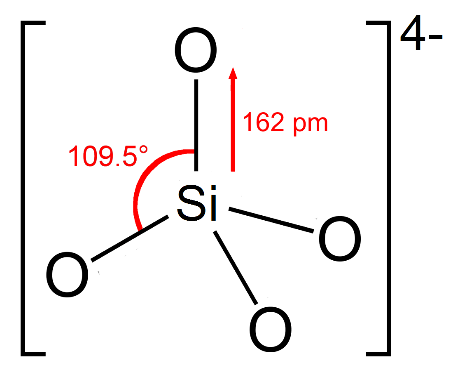
And if some of the silicon atoms are in a three dimensional network then the silicates are replaced by $A{l^{3 + }}$ ions, the overall structure carries a negative charge which is known as aluminum silicate. These three-dimensional structures are called feldspar and zeolites.
Complete step by step answer:
We know that water develops interlocking needles like crystals of hydrated silicates. This reaction involves the hydration of calcium aluminates and calcium silicates which change into their colloidal gels.
At the same time, some calcium hydroxide and aluminum hydroxides are formed as precipitates due to hydrolysis. Calcium hydroxide binds the particles of calcium silicate together while aluminum hydroxide fills the interstices rendering the mass impervious.
Therefore, we can conclude that in curing cement plasters water is sprinkled from time to times to develop interlocking needle-like crystals of hydrated silicates.
Therefore, the option A is correct (developing interlocking needle-like crystals of hydrated silicates).
Note:
For solving this question, we also need to know about the structure of silicates. Silicates are known to show tetrahedral structure in which small open circles represent oxygen atoms and the small closed circle inside represent the silicon atom. We can draw the structure of silicate as given below,

And if some of the silicon atoms are in a three dimensional network then the silicates are replaced by $A{l^{3 + }}$ ions, the overall structure carries a negative charge which is known as aluminum silicate. These three-dimensional structures are called feldspar and zeolites.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




