
In column chromatography stationary phase is:
A) Only solid.
B) Only liquid.
C) Only gas.
D) Any one of these.
Answer
583.2k+ views
Hint:We all know that chromatography may be a technique which is employed to separate one compound from a mix which is dissolved during a fluid. During this technique the substances are separated depending upon their adsorption of compounds to the adsorbent if the compounds are skilled at the column at different rates which permit them to urge separated infractions.
Complete step by step answer:
If the mobile phase alongside the mixture which needs to be separated is passed from the highest of the column, the individual components of the mixture move at different rates. The components with lower adsorption and affinity to stationary phase travel faster in comparison to the components which have greater adsorption and affinity with the stationary phase. The components that move fast are removed first whereas the components that crawl are eluted out last. The adsorption of solute molecules to the column occurs during a reversible manner. The speed of the movement of the components is expressed as it's the space travelled by solute is named the retardation factor.
The model diagram is given below which shows how the column is prepared.
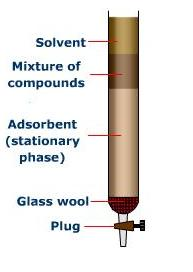
Adsorbent is the stationary phase in column chromatography. Adsorbents used are silica gel alumina both are solids. The selection of stationary phase should obey following properties and they are listed below as,
Uniform shape and size range should be $60 - 200\mu $ in diameter.
Inexpensive and must be readily available.
Should be chemically inert and having good adsorption property.
Therefore, the option A is correct.
Note: We must remember that column chromatography systems are often used on a little scale also as large scale to purify materials which will be utilized in future experiments. This method may be a sort of adsorption chromatography technique.
Active ingredients are isolated by column chromatography and also used for the estimation of drugs.
Complete step by step answer:
If the mobile phase alongside the mixture which needs to be separated is passed from the highest of the column, the individual components of the mixture move at different rates. The components with lower adsorption and affinity to stationary phase travel faster in comparison to the components which have greater adsorption and affinity with the stationary phase. The components that move fast are removed first whereas the components that crawl are eluted out last. The adsorption of solute molecules to the column occurs during a reversible manner. The speed of the movement of the components is expressed as it's the space travelled by solute is named the retardation factor.
The model diagram is given below which shows how the column is prepared.

Adsorbent is the stationary phase in column chromatography. Adsorbents used are silica gel alumina both are solids. The selection of stationary phase should obey following properties and they are listed below as,
Uniform shape and size range should be $60 - 200\mu $ in diameter.
Inexpensive and must be readily available.
Should be chemically inert and having good adsorption property.
Therefore, the option A is correct.
Note: We must remember that column chromatography systems are often used on a little scale also as large scale to purify materials which will be utilized in future experiments. This method may be a sort of adsorption chromatography technique.
Active ingredients are isolated by column chromatography and also used for the estimation of drugs.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




