
In ccp structure, the
A.The first and third layers are repeated.
B.The first and fourth layers are repeated.
D.The first, third and sixth layers are repeated.
C.The second and fourth layers are repeated.
Answer
509.7k+ views
Hint : We know that ccp’ arrangement stands for cubic close packing arrangement. The arrangement in a cubic close packing is such that its packing efficiency is \[74\] percent. For such types of problems, you need high imagination skills in three dimensions. But the best approach can be to try to think of all layers individually and then try to figure out patterns.
Complete Step By Step Answer:
The ccp arrangement is obtained when the third layer of a structure is: First and fourth layer is the same.
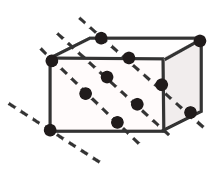
Now when the third layer is placed over the second, there are only two possibilities. First possibility by covering tetrahedral voids and the second by covering octahedral voids. -First, we will understand tetrahedral voids and octahedral voids. So tetrahedral voids are formed when the sphere of the second layer is above the void of the first layer.
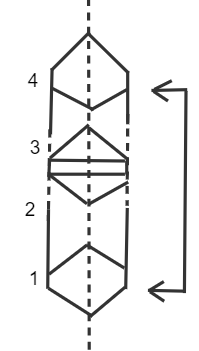
Octahedral voids are present at the edge Centre and body Centre. -When the third layer is placed over the second by covering tetrahedral voids then in this possibility the sphere of the third layer will be symmetric with the first layer and if we follow the pattern the spheres of the second layer will be aligned with the fourth layer and so on. The same layer diagram is given below:
Therefore, the correct answer is Option B.
Note :
Remember that there is a very important point to remember in the ABC ABC ABC ….... pattern and that is if spheres of the third layer are placed into tetrahedral voids, the packing is hcp (hexagonal close packing). CCP is cubic close packing, it is also called fcc (face-centered cubic). In fcc (face-centered cubic), spheres of the third layer are placed into octahedral voids.
Complete Step By Step Answer:
The ccp arrangement is obtained when the third layer of a structure is: First and fourth layer is the same.

Now when the third layer is placed over the second, there are only two possibilities. First possibility by covering tetrahedral voids and the second by covering octahedral voids. -First, we will understand tetrahedral voids and octahedral voids. So tetrahedral voids are formed when the sphere of the second layer is above the void of the first layer.
Octahedral voids are present at the edge Centre and body Centre. -When the third layer is placed over the second by covering tetrahedral voids then in this possibility the sphere of the third layer will be symmetric with the first layer and if we follow the pattern the spheres of the second layer will be aligned with the fourth layer and so on. The same layer diagram is given below:

Therefore, the correct answer is Option B.
Note :
Remember that there is a very important point to remember in the ABC ABC ABC ….... pattern and that is if spheres of the third layer are placed into tetrahedral voids, the packing is hcp (hexagonal close packing). CCP is cubic close packing, it is also called fcc (face-centered cubic). In fcc (face-centered cubic), spheres of the third layer are placed into octahedral voids.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




