
In carbonyl group oxygen has higher electronegativity which make it:
A: Polarized
B: non-polarized
C: stable
D: unstable
Answer
582.9k+ views
Hint: In the carbonyl group the carbon atom has a double bond with the oxygen atom. The oxygen atom has more electronegativity than the carbon atom. The oxygen atom tries to withdraw electrons making the carbon atom an electrophilic centre.
Complete step by step answer:
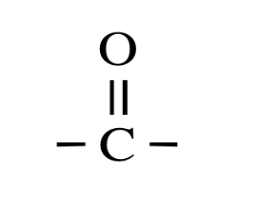
As we know the electronegativity of an oxygen atom is greater than that of a carbon atom. So the oxygen tries to withdraw or attract the electrons from the carbon atom. This induces a partial positive charge in the carbon atom and a partial negative charge in the oxygen atom. This makes the entire molecule a polar molecule. The carbonyl group is polar in nature.
So, the correct answer is Option A.
Additional Information:
Carbonyl group acts as an excellent nucleophilic centre . The nucleophiles attack the carbon atom for nucleophilic addition reaction. Carbonyl groups can be found in different functional groups such as ketone, aldehyde, ester and many more. The carbonyl group increases the boiling point of the compound. The carbonyl groups act as an electron withdrawing group. This makes the carbonyl group very reactive and they undergo many reactions. Due to the high electron withdrawing characteristic of the carbonyl group , they often have resonance structures which affect their reactivity .The carbonyl group undergoes some reactions which are very much important to sustain life. Carbonyl groups allow the cells to react with various functional groups and modify itself.
Note:
The electronegativity of oxygen atoms is $3.44$. The electronegativity of the carbon atom is $2.55$. This is the main reason which makes the carbonyl group a polar group. The difference of electronegativity decides whether a compound is polar or not.
Complete step by step answer:

As we know the electronegativity of an oxygen atom is greater than that of a carbon atom. So the oxygen tries to withdraw or attract the electrons from the carbon atom. This induces a partial positive charge in the carbon atom and a partial negative charge in the oxygen atom. This makes the entire molecule a polar molecule. The carbonyl group is polar in nature.
So, the correct answer is Option A.
Additional Information:
Carbonyl group acts as an excellent nucleophilic centre . The nucleophiles attack the carbon atom for nucleophilic addition reaction. Carbonyl groups can be found in different functional groups such as ketone, aldehyde, ester and many more. The carbonyl group increases the boiling point of the compound. The carbonyl groups act as an electron withdrawing group. This makes the carbonyl group very reactive and they undergo many reactions. Due to the high electron withdrawing characteristic of the carbonyl group , they often have resonance structures which affect their reactivity .The carbonyl group undergoes some reactions which are very much important to sustain life. Carbonyl groups allow the cells to react with various functional groups and modify itself.
Note:
The electronegativity of oxygen atoms is $3.44$. The electronegativity of the carbon atom is $2.55$. This is the main reason which makes the carbonyl group a polar group. The difference of electronegativity decides whether a compound is polar or not.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




