
In biological systems, some amino acids are more soluble in water due to the hydrophilic regions. Based on the structure of amino acids pictured, which of the following would be LEAST soluble in water?
A.Histidine
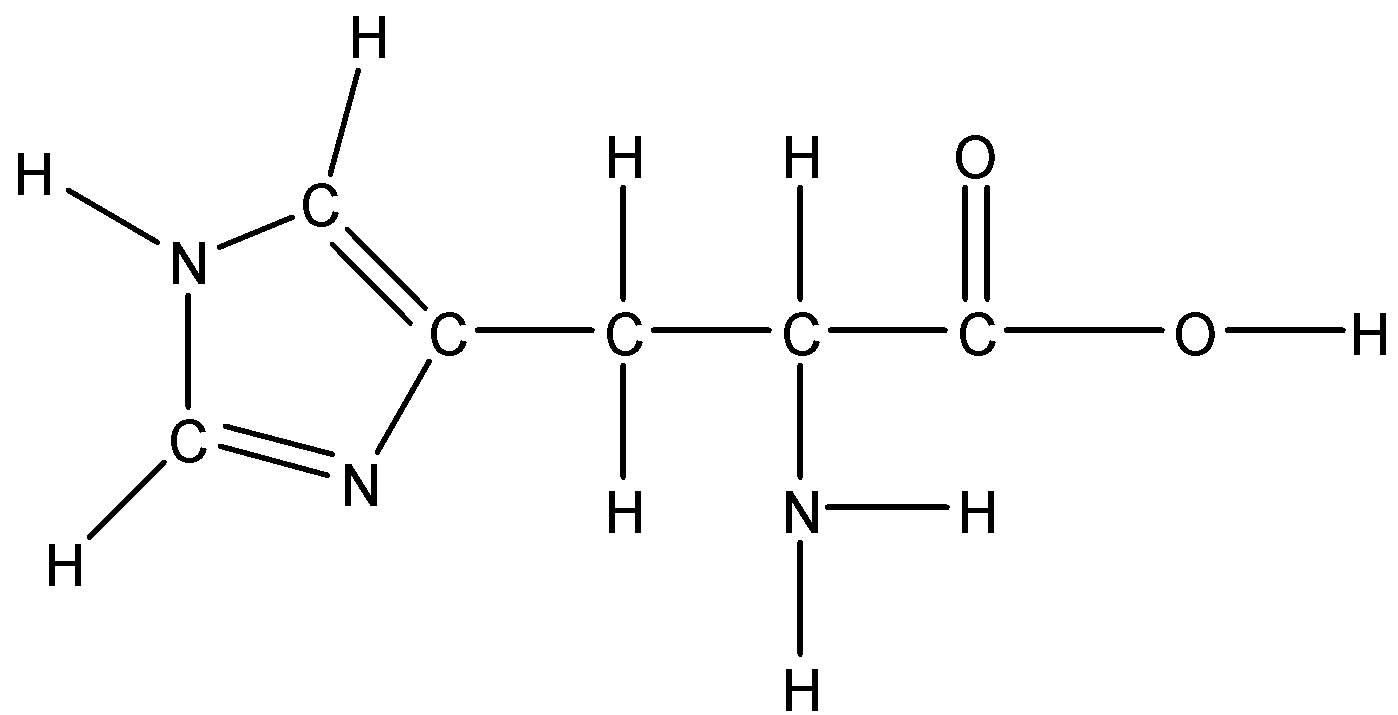
B.Lysine
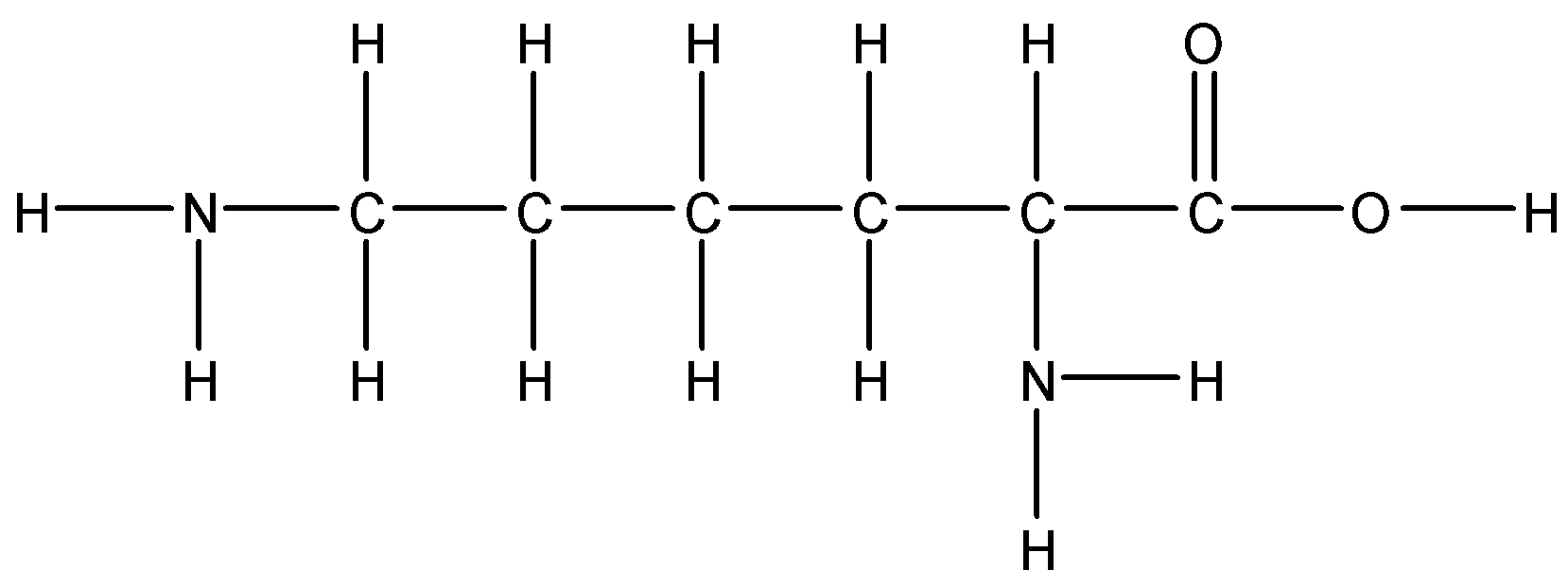
C.Alanine
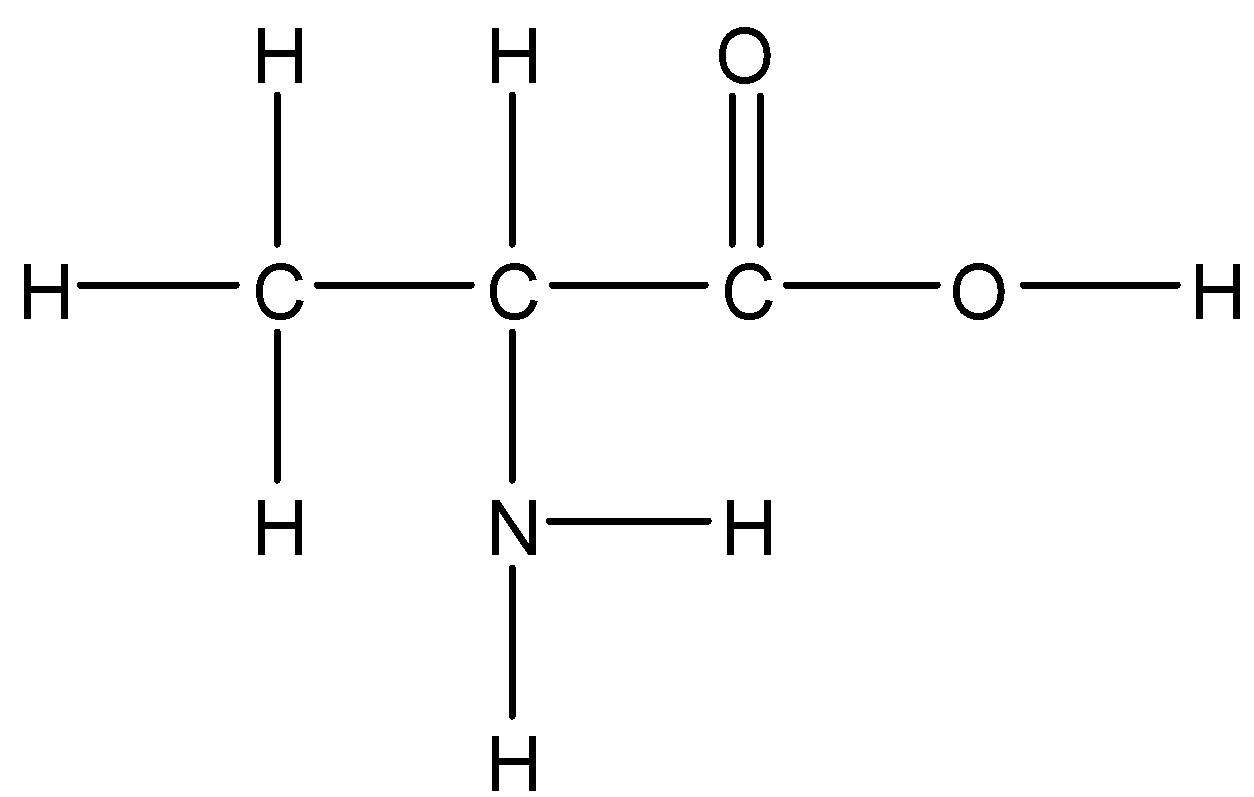
D.Cysteine
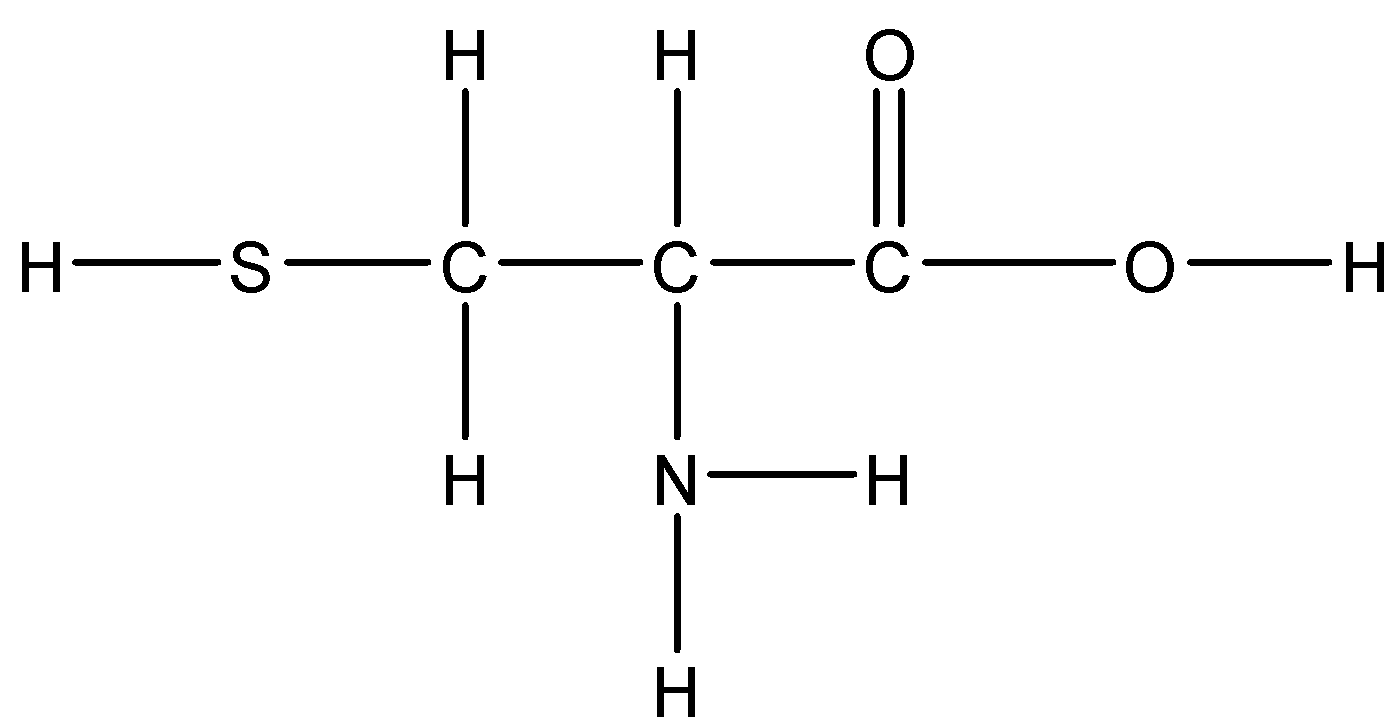




Answer
565.5k+ views
Hint:Amino acids are organic compounds that contain amine and carboxyl functional groups along with a side chain specific to each amino acid. Amino acids are usually classified by the properties of their side chain into four groups. The side chain can make an amino acid a weak acid or a weak base, a hydrophile if the side chain is polar, or hydrophobic if it is nonpolar. In an aqueous solution, amino acids exist in two forms, the molecular form and the zwitterion form in equilibrium with each other. Amino acids are generally soluble in water and insoluble in nonpolar organic solvents such as hydrocarbons.
Complete step by step answer:
Let us discuss the given options one by one.
Option A: Histidine
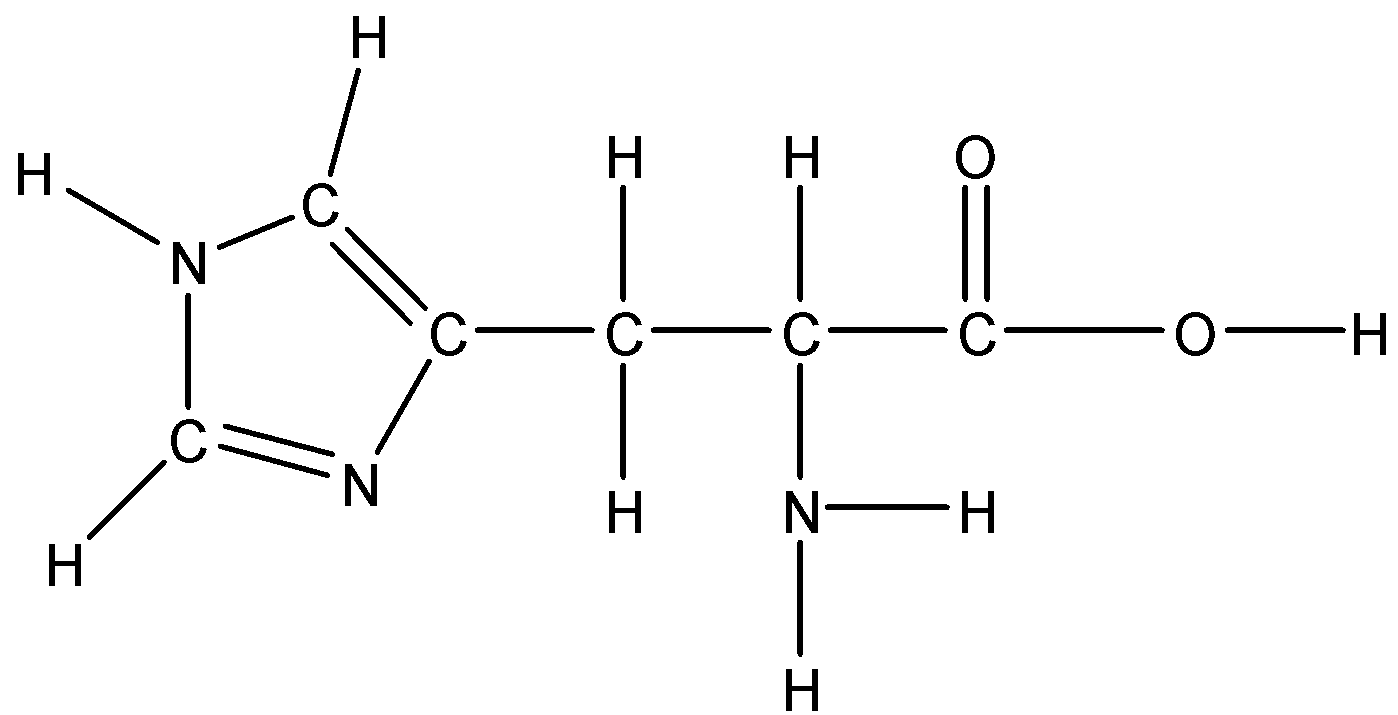
Histidine is a basic amino acid. Since it has a nonpolar aromatic ring in its side chain so it is not soluble in water because water is polar and we know that like dissolves like. The ring is not polar.
Option B: Lysine
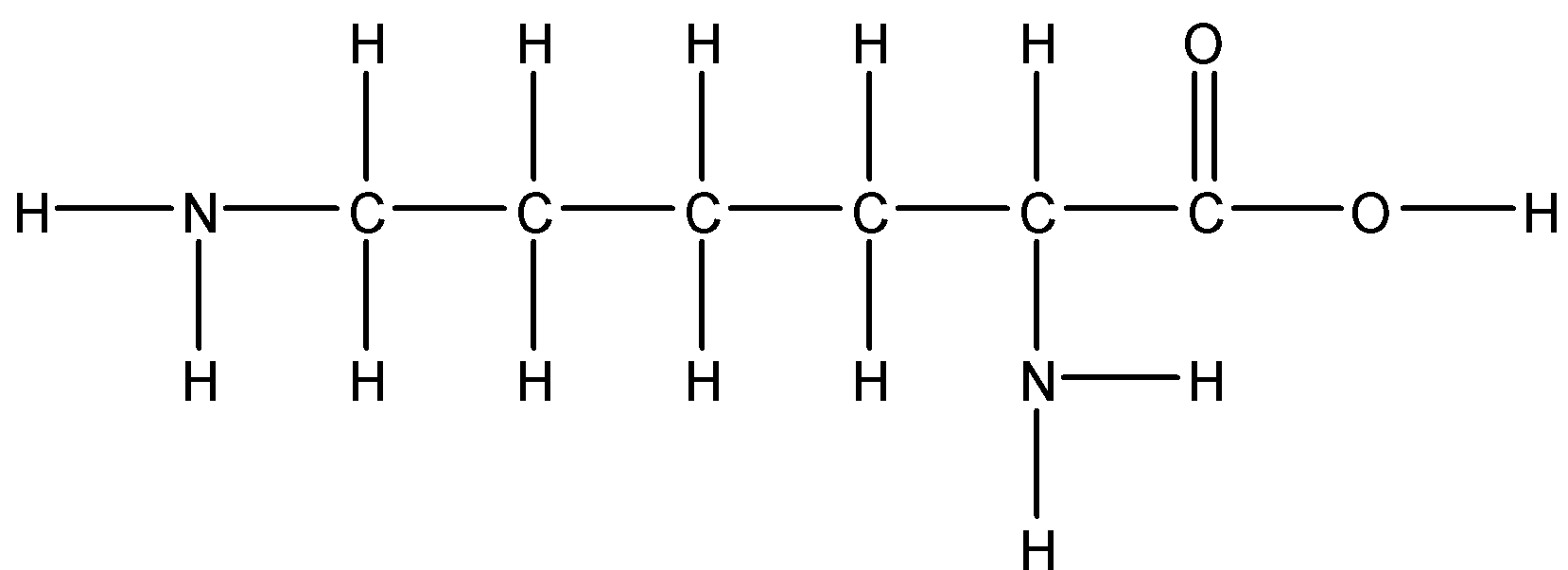
Lysine is also a basic amino acid. Since it has a polar side chain it is soluble in water.
Option C: Alanine
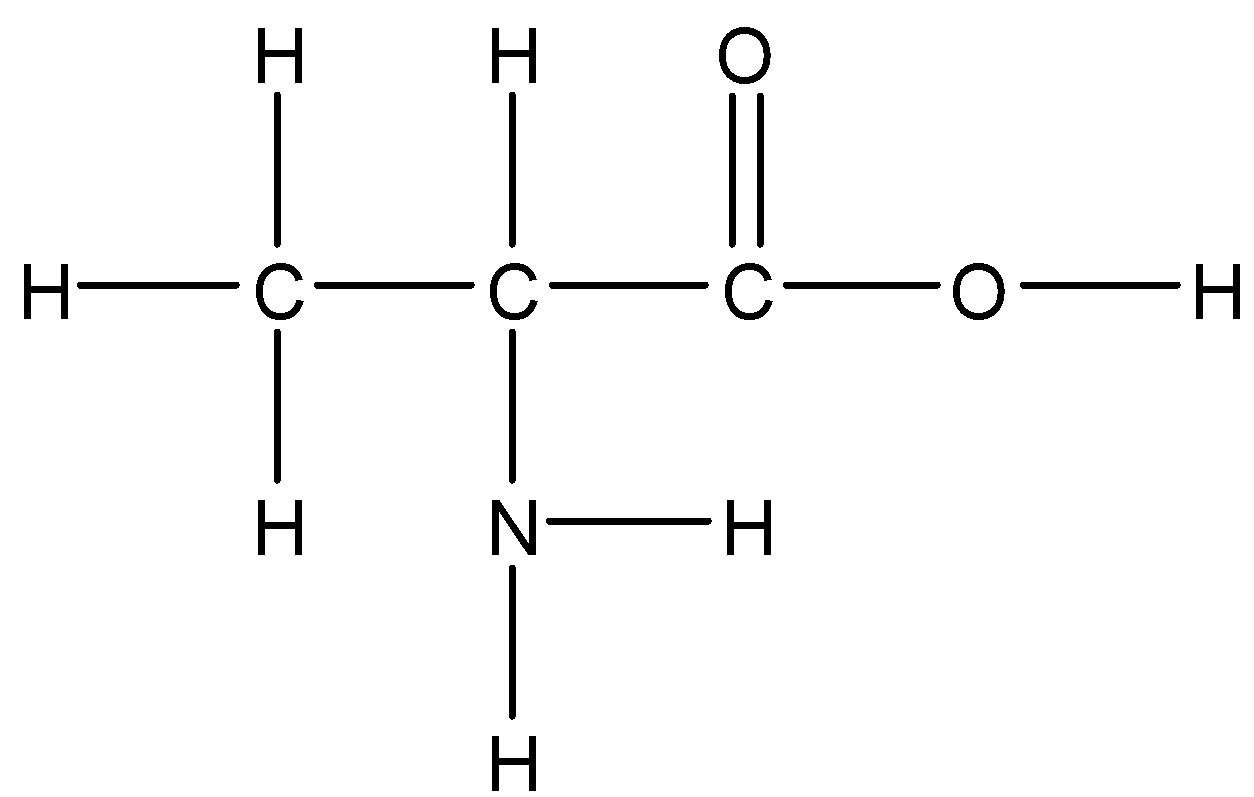
Alanine is a neutral amino acid. It has a polar side chain so it is soluble in water.
Option D: Cysteine
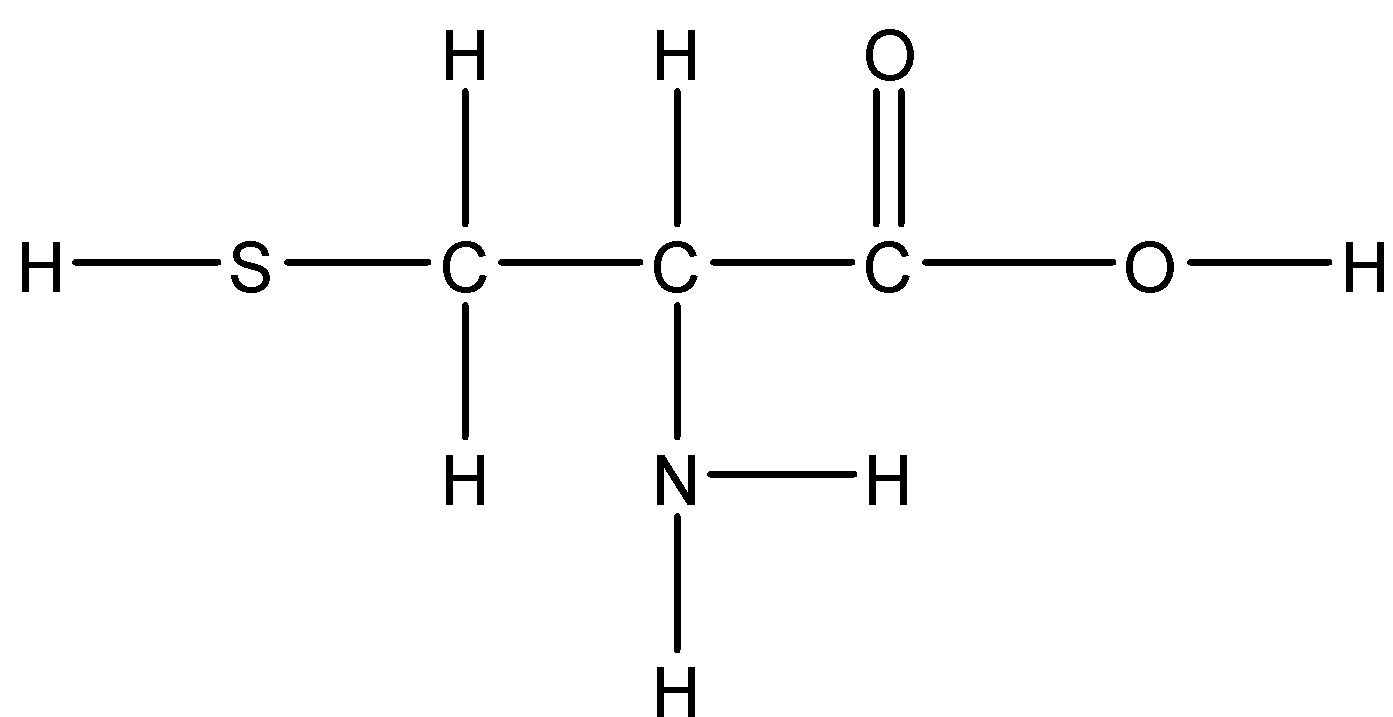
Cysteine has a slightly polar \[S - H\] which has a slight polarity. Hence it is mildly soluble in water.
Thus the correct option is A.
Note:
The polarity or non-polarity of an amino acid depends upon the \[ - R\] group present in its side chain. Aromatic rings are non-polar.
Histidine is an essential amino acid and is present in many proteins. Its decarboxylation gives histamine.
Lysine is also an essential amino acid and is important for normal growth.
The polarity of amino acids affects the structures of proteins formed by it.
All polar amino acids can make hydrogen bonds with suitable groups.
Complete step by step answer:
Let us discuss the given options one by one.
Option A: Histidine

Histidine is a basic amino acid. Since it has a nonpolar aromatic ring in its side chain so it is not soluble in water because water is polar and we know that like dissolves like. The ring is not polar.
Option B: Lysine

Lysine is also a basic amino acid. Since it has a polar side chain it is soluble in water.
Option C: Alanine

Alanine is a neutral amino acid. It has a polar side chain so it is soluble in water.
Option D: Cysteine

Cysteine has a slightly polar \[S - H\] which has a slight polarity. Hence it is mildly soluble in water.
Thus the correct option is A.
Note:
The polarity or non-polarity of an amino acid depends upon the \[ - R\] group present in its side chain. Aromatic rings are non-polar.
Histidine is an essential amino acid and is present in many proteins. Its decarboxylation gives histamine.
Lysine is also an essential amino acid and is important for normal growth.
The polarity of amino acids affects the structures of proteins formed by it.
All polar amino acids can make hydrogen bonds with suitable groups.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




