
In ammoniacal solution, dichromate in presence of H202 forms ${{\text{(N}{{\text{H}}_{\text{3}}}\text{)}}_{\text{3}}}\text{Cr}{{\text{O}}_{\text{4}}}$ in which \[\text{Cr}\] is in $\text{IV}$ the state. The structure is a pentagonal bipyramid. State true or false.
A) True
B) False
Answer
589.8k+ views
Hint: This ${{\text{(N}{{\text{H}}_{\text{3}}}\text{)}}_{\text{3}}}\text{Cr}{{\text{O}}_{\text{4}}}$ is a pentagonal bipyramidal structure. It involves the peroxo linkages. The chromium is tetravalent. It is synthesized from the dichromate in presence of $30{\scriptstyle{}^{0}/{}_{0}}$${{\text{H}}_{\text{2}}}{{\text{O}}_{\text{2}}}$.
Complete answer:
It always mentioned that only a considerable number of peroxo-chromate complexes are known. These compounds are more or less stable and these peroxo compounds decompose slowly with the evolution of oxygen. Some of these peroxo compounds are explosive.
This ${{\text{(N}{{\text{H}}_{\text{3}}}\text{)}}_{\text{3}}}\text{Cr}{{\text{O}}_{\text{4}}}$ is synthesized by the treatment of alkaline dichromate solutions $30{\scriptstyle{}^{0}/{}_{0}}$ ${{\text{H}}_{\text{2}}}{{\text{O}}_{\text{2}}}$. This forms the peroxo chromate$\text{(CrO}_{\text{8}}^{\text{3-}}\text{)}$. They are paramagnetic with the once unoccupied electron per formula unit.
We can say that in $\text{(CrO}_{\text{8}}^{\text{3-}}\text{)}$ the chromium is pentavalent.
When the mixture used for the preparation of ${{\text{(N}{{\text{H}}_{\text{4}}}\text{)}}_{\text{3}}}\text{Cr}{{\text{O}}_{\text{8}}}$ is heated at the ${{50}^{0}}C$ and cooled down to ${{0}^{0}}C$ a brown crystal of ${{\text{(N}{{\text{H}}_{4}}\text{)}}_{\text{3}}}\text{Cr}{{\text{O}}_{\text{4}}}$ are obtained-ray studies has revealed the structure of ${{\text{(N}{{\text{H}}_{\text{3}}}\text{)}}_{\text{3}}}\text{Cr}{{\text{O}}_{\text{4}}}$ as shown below:
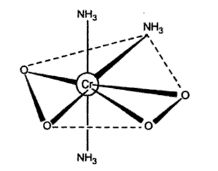
The ${{\text{(N}{{\text{H}}_{\text{3}}}\text{)}}_{\text{3}}}\text{Cr}{{\text{O}}_{\text{4}}}$ has the central metal atom as the chromium. The central chromium ion is surrounded by a total of seven ligands around it. The three are ammonia groups $\text{(N}{{\text{H}}_{\text{3}}}\text{)}$ and four of the oxygen are arranged around the central ion. The two superoxide ligands are bonded to the chromium. There are a total of five coordinating sites. For five ligands the possible structure is pentagonal bipyramidal since each oxygen of superoxide is considered as a single ligand.
Here, the oxidation state of chromium is a low valence state. The divalent chromium is coordinated to the two superoxide $\text{(-O-O-)}$ ions. The compound contains the two unpaired electrons and thus chromium is diamagnetic. In other words, we can naturally consider a compound containing the tetravalent chromium $\text{IV}$ which is coordinated peroxide ions.
Hence, when the ammoniacal solution, dichromate in presence of ${{\text{H}}_{\text{2}}}{{\text{O}}_{\text{2}}}$ forms ${{\text{(N}{{\text{H}}_{\text{3}}}\text{)}}_{\text{3}}}\text{Cr}{{\text{O}}_{\text{4}}}$ in which $\text{Cr}$ Is in $\text{IV}$ the state. The structure is a pentagonal bipyramid.
Hence, the statement is true. Correct option (A).
Note:
There are various peroxy –chromate for example deep blue chromium peroxide $\text{(Cr}{{\text{O}}_{\text{5}}}\text{)}$, the blue peroxy-chromate $\text{C}{{\text{r}}_{\text{2}}}\text{O}_{\text{12}}^{\text{2-}}$ , and the red peroxy-chromate $\text{(CrO}_{\text{8}}^{\text{3-}})$ .
Complete answer:
It always mentioned that only a considerable number of peroxo-chromate complexes are known. These compounds are more or less stable and these peroxo compounds decompose slowly with the evolution of oxygen. Some of these peroxo compounds are explosive.
This ${{\text{(N}{{\text{H}}_{\text{3}}}\text{)}}_{\text{3}}}\text{Cr}{{\text{O}}_{\text{4}}}$ is synthesized by the treatment of alkaline dichromate solutions $30{\scriptstyle{}^{0}/{}_{0}}$ ${{\text{H}}_{\text{2}}}{{\text{O}}_{\text{2}}}$. This forms the peroxo chromate$\text{(CrO}_{\text{8}}^{\text{3-}}\text{)}$. They are paramagnetic with the once unoccupied electron per formula unit.
We can say that in $\text{(CrO}_{\text{8}}^{\text{3-}}\text{)}$ the chromium is pentavalent.
When the mixture used for the preparation of ${{\text{(N}{{\text{H}}_{\text{4}}}\text{)}}_{\text{3}}}\text{Cr}{{\text{O}}_{\text{8}}}$ is heated at the ${{50}^{0}}C$ and cooled down to ${{0}^{0}}C$ a brown crystal of ${{\text{(N}{{\text{H}}_{4}}\text{)}}_{\text{3}}}\text{Cr}{{\text{O}}_{\text{4}}}$ are obtained-ray studies has revealed the structure of ${{\text{(N}{{\text{H}}_{\text{3}}}\text{)}}_{\text{3}}}\text{Cr}{{\text{O}}_{\text{4}}}$ as shown below:

The ${{\text{(N}{{\text{H}}_{\text{3}}}\text{)}}_{\text{3}}}\text{Cr}{{\text{O}}_{\text{4}}}$ has the central metal atom as the chromium. The central chromium ion is surrounded by a total of seven ligands around it. The three are ammonia groups $\text{(N}{{\text{H}}_{\text{3}}}\text{)}$ and four of the oxygen are arranged around the central ion. The two superoxide ligands are bonded to the chromium. There are a total of five coordinating sites. For five ligands the possible structure is pentagonal bipyramidal since each oxygen of superoxide is considered as a single ligand.
Here, the oxidation state of chromium is a low valence state. The divalent chromium is coordinated to the two superoxide $\text{(-O-O-)}$ ions. The compound contains the two unpaired electrons and thus chromium is diamagnetic. In other words, we can naturally consider a compound containing the tetravalent chromium $\text{IV}$ which is coordinated peroxide ions.
Hence, when the ammoniacal solution, dichromate in presence of ${{\text{H}}_{\text{2}}}{{\text{O}}_{\text{2}}}$ forms ${{\text{(N}{{\text{H}}_{\text{3}}}\text{)}}_{\text{3}}}\text{Cr}{{\text{O}}_{\text{4}}}$ in which $\text{Cr}$ Is in $\text{IV}$ the state. The structure is a pentagonal bipyramid.
Hence, the statement is true. Correct option (A).
Note:
There are various peroxy –chromate for example deep blue chromium peroxide $\text{(Cr}{{\text{O}}_{\text{5}}}\text{)}$, the blue peroxy-chromate $\text{C}{{\text{r}}_{\text{2}}}\text{O}_{\text{12}}^{\text{2-}}$ , and the red peroxy-chromate $\text{(CrO}_{\text{8}}^{\text{3-}})$ .
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




