
In alkaline hydrolysis of tertiary halide by aqueous alkali if concentration of alkali doubled, then the reaction:
(A) will be doubled
(B) will be halved
(C) will remain constant
(D) None of the above
Answer
594.6k+ views
Hint: Alkaline hydrolysis of a tertiary halide is a \[{S_N}1\] type of reaction. In tertiary halide, there are three carbon atoms directly bonded with the carbon that bears the halogen atom.
Complete answer:
In the alkaline hydrolysis of tertiary halide by aqueous alkali, the type of reaction will determine whether it depends on the concentration of alkali or not.
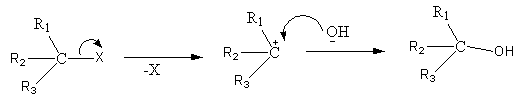
Suppose that a tertiary halide reacts in given conditions. Now there are two possible pathways the reaction can follow, \[{S_N}1\] or \[{S_N}2\]. We know that tertiary halides can not undergo substitution reaction by \[{S_N}2\] mechanism. The reason is that they have a very crowded back side, hence back side attack is not possible in their cases.
- Inversely, \[{S_N}1\] mechanism is favoured in tertiary halides and hence this reaction will also follow the\[{S_N}1\] pathway.
- As the name suggests, in \[{S_N}1\] mechanism the reaction rate only depends upon the concentration of the reactant means the tertiary halide and the raet does not depend upon the concentration of alkali at all. So, as the concentration of alkali changes it will not have any effect on the reaction rate.
So, correct answer is (C) will remain constant.
Additional Information:
If primary halide was given in the question in place of tertiary, then it would follow \[{S_N}2\] type of mechanism and it would be dependent on the concentration of alkali. So, in that case if the concentration of the alkali would have been doubled then, the rate of the reaction would also get doubled.
Note: Do not just blindly think that as the concentration of the reagent alkali is doubled, the rate will also be doubled, certain reactions like \[{S_N}1\] do not get affected by attacking reagent. Do not forget the stability order of carbocations that is useful everywhere in organic chemistry which is Tertiary < Secondary < Primary.
Complete answer:
In the alkaline hydrolysis of tertiary halide by aqueous alkali, the type of reaction will determine whether it depends on the concentration of alkali or not.

Suppose that a tertiary halide reacts in given conditions. Now there are two possible pathways the reaction can follow, \[{S_N}1\] or \[{S_N}2\]. We know that tertiary halides can not undergo substitution reaction by \[{S_N}2\] mechanism. The reason is that they have a very crowded back side, hence back side attack is not possible in their cases.
- Inversely, \[{S_N}1\] mechanism is favoured in tertiary halides and hence this reaction will also follow the\[{S_N}1\] pathway.
- As the name suggests, in \[{S_N}1\] mechanism the reaction rate only depends upon the concentration of the reactant means the tertiary halide and the raet does not depend upon the concentration of alkali at all. So, as the concentration of alkali changes it will not have any effect on the reaction rate.
So, correct answer is (C) will remain constant.
Additional Information:
If primary halide was given in the question in place of tertiary, then it would follow \[{S_N}2\] type of mechanism and it would be dependent on the concentration of alkali. So, in that case if the concentration of the alkali would have been doubled then, the rate of the reaction would also get doubled.
Note: Do not just blindly think that as the concentration of the reagent alkali is doubled, the rate will also be doubled, certain reactions like \[{S_N}1\] do not get affected by attacking reagent. Do not forget the stability order of carbocations that is useful everywhere in organic chemistry which is Tertiary < Secondary < Primary.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




