
In acids, Methyl orange turns.
A) Yellow
B) Green
C) red
D) White
Answer
579.9k+ views
Hint: We define \[pH\] indicators are weak acids that exist as natural dyes and indicate the concentration of \[{H^ + }\] ions during a solution via color change.
Complete step by step answer:
We know that the chemical formula of methyl orange is \[{C_{14}}{H_{14}}{N_3}Na{O_3}S\]. Methyl orange shows yellow color when the \[pH\] is above \[4.4\]. Methyl orange in xylene cyanol solution when \[pH\] above \[4.2\] shows green colors. The above two ranges lie in pH above 4 so that solution is less acidic. Methyl orange shows red colors when the solution of \[pH\] is \[3.1\] it means acidic.
In alkali solution, methyl orange gives yellow colour and in an acidic solution, H+ ions approaches the one of nitrogen in N=N bond to give a red coloured solution. The structure of methyl orange in acid and alkali solution is given below as,
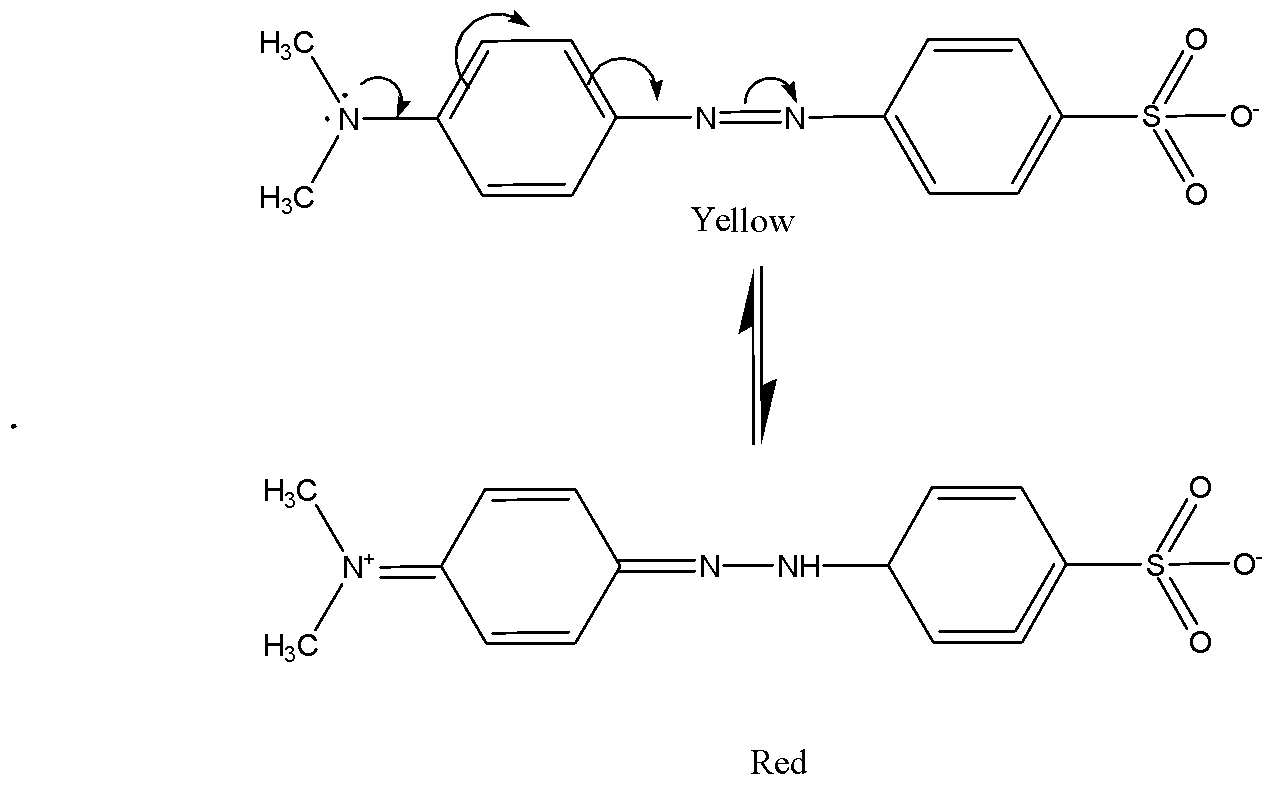
So, the correct answer is Option C.
Note:
We must remember that the universal indicator is a brown colored solution which has a mix of indicators, which will be added to any substance to work out its\[pH\]. It changes color in different pH environments. At the \[pH\] range of \[8 - 11\] , the color is blue.
we discuss about the type of universal indicator as,
Paper form: It is a stripe of colored paper which changes color of the solution to red if the solution is acidic and changes the color of solution to blue, if the solution is basic. Using dropping equipment a drop of test solution is placed on the strip. We can use the paper universal indicator for the dark coloured test solution.
Complete step by step answer:
We know that the chemical formula of methyl orange is \[{C_{14}}{H_{14}}{N_3}Na{O_3}S\]. Methyl orange shows yellow color when the \[pH\] is above \[4.4\]. Methyl orange in xylene cyanol solution when \[pH\] above \[4.2\] shows green colors. The above two ranges lie in pH above 4 so that solution is less acidic. Methyl orange shows red colors when the solution of \[pH\] is \[3.1\] it means acidic.
In alkali solution, methyl orange gives yellow colour and in an acidic solution, H+ ions approaches the one of nitrogen in N=N bond to give a red coloured solution. The structure of methyl orange in acid and alkali solution is given below as,

So, the correct answer is Option C.
Note:
We must remember that the universal indicator is a brown colored solution which has a mix of indicators, which will be added to any substance to work out its\[pH\]. It changes color in different pH environments. At the \[pH\] range of \[8 - 11\] , the color is blue.
we discuss about the type of universal indicator as,
Paper form: It is a stripe of colored paper which changes color of the solution to red if the solution is acidic and changes the color of solution to blue, if the solution is basic. Using dropping equipment a drop of test solution is placed on the strip. We can use the paper universal indicator for the dark coloured test solution.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




