
In a weak acidic medium, the electrolytic reduction of nitrobenzene will give which of the following products?
A. aniline
B. nitrobenzene
C. phenylhydroxylamine
D. p-aminophenol
Answer
560.7k+ views
Hint: We know that amines can be prepared by reduction of nitro compounds. The catalysts used in this reduction reaction are palladium, platinum in presence of hydrogen gas.
Complete step by step answer:
Let’s discuss reduction of nitro compounds in detail. Amines can be obtained by passing hydrogen gas in the presence of finely divided palladium, nickel or platinum. Another way of producing amine is by reducing nitro compounds by metal in the acidic medium. The reduction reaction can be shown as below.
1st type (Reduction by hydrogen in presence of nickel, palladium or platinum)
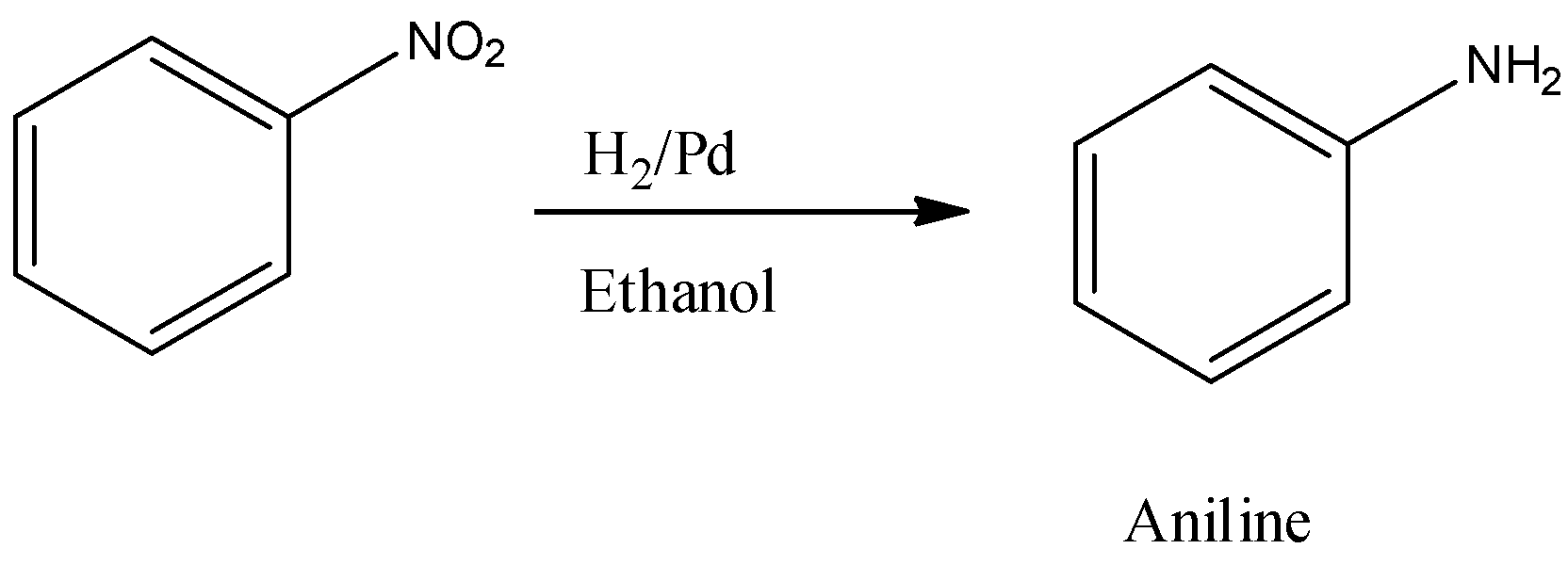
2nd type (Reduction by metals in presence of acid)
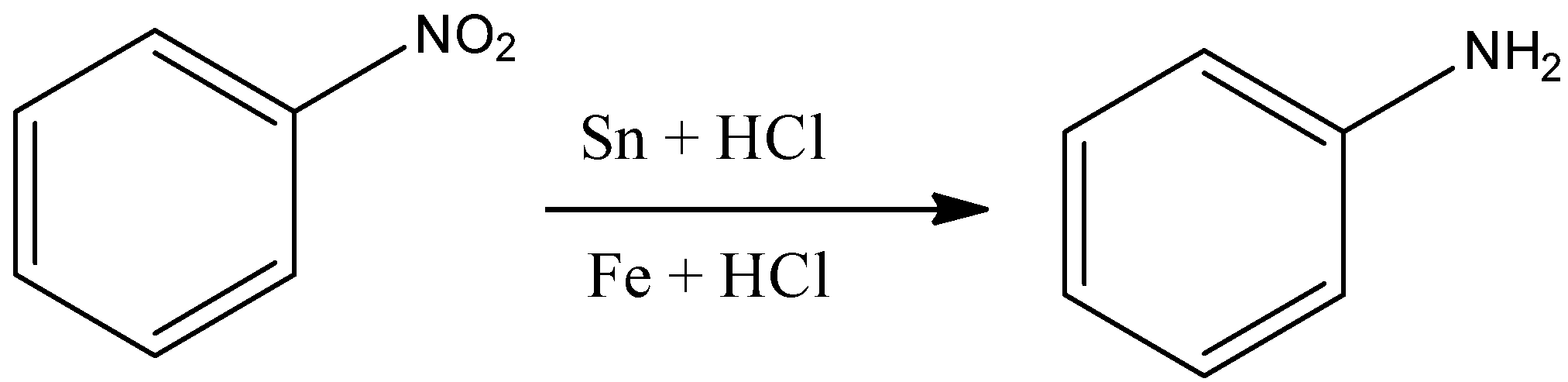
Now, come to the question. We have to identify the product that is obtained on reducing the nitro compound in a weak acidic medium.
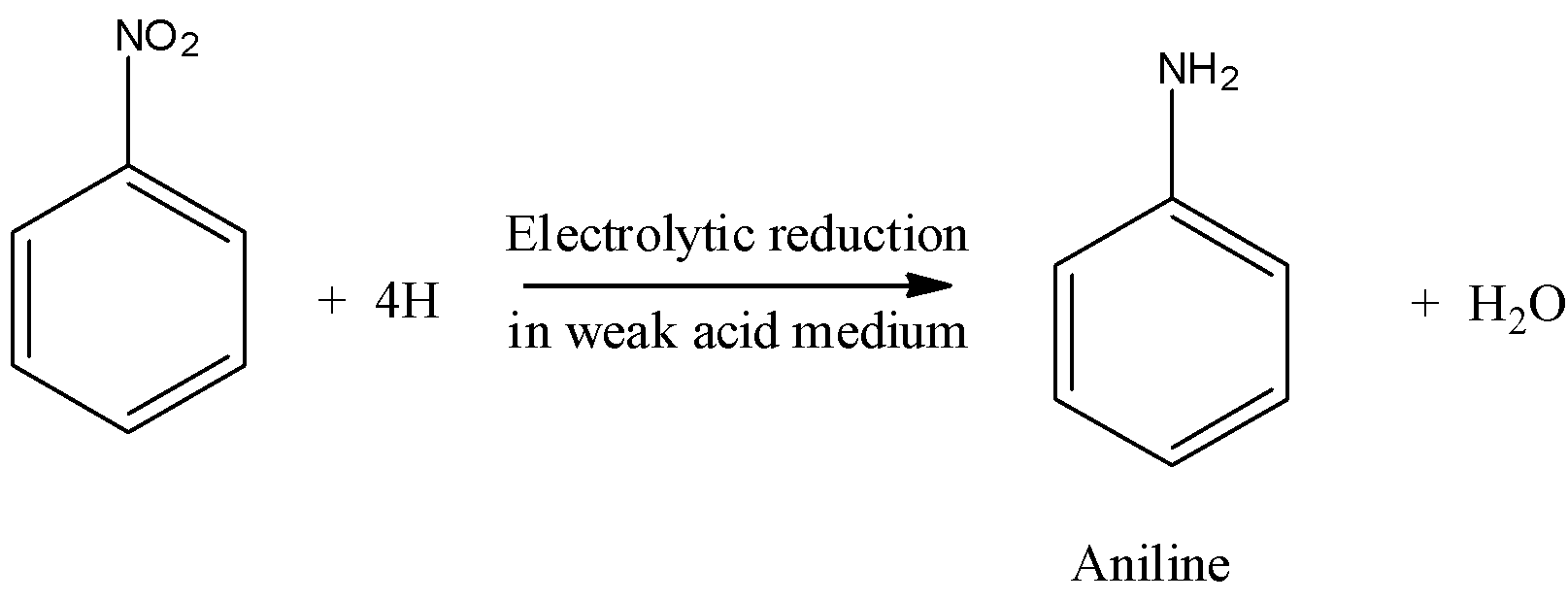
So, the product obtained in aniline.
So, the correct answer is Option A.
Additional Information:
Another way to produce amines is by reducing nitriles. When nitriles undergo reduction with lithium aluminium hydride $\left( {{\rm{LiAl}}{{\rm{H}}_{\rm{4}}}} \right)$ or catalytic hydrogenation produces primary amines. This reaction is used to increase the number of carbon atoms by one than the starting amine. The reaction can be shown as below.

Note: It is to be noted that in case of reduction by metal in presence of a strong acid, the reduction with iron scrap (Fe) and hydrochloric acid (HCl) is preferred than Sn and HCl because ${\rm{FeC}}{{\rm{l}}_{\rm{3}}}$ formed gets hydrolysed and releases hydrochloric acid during the course of reaction. Therefore, only a small amount of hydrochloric acid is required to initiate the reaction.
Complete step by step answer:
Let’s discuss reduction of nitro compounds in detail. Amines can be obtained by passing hydrogen gas in the presence of finely divided palladium, nickel or platinum. Another way of producing amine is by reducing nitro compounds by metal in the acidic medium. The reduction reaction can be shown as below.
1st type (Reduction by hydrogen in presence of nickel, palladium or platinum)

2nd type (Reduction by metals in presence of acid)

Now, come to the question. We have to identify the product that is obtained on reducing the nitro compound in a weak acidic medium.

So, the product obtained in aniline.
So, the correct answer is Option A.
Additional Information:
Another way to produce amines is by reducing nitriles. When nitriles undergo reduction with lithium aluminium hydride $\left( {{\rm{LiAl}}{{\rm{H}}_{\rm{4}}}} \right)$ or catalytic hydrogenation produces primary amines. This reaction is used to increase the number of carbon atoms by one than the starting amine. The reaction can be shown as below.

Note: It is to be noted that in case of reduction by metal in presence of a strong acid, the reduction with iron scrap (Fe) and hydrochloric acid (HCl) is preferred than Sn and HCl because ${\rm{FeC}}{{\rm{l}}_{\rm{3}}}$ formed gets hydrolysed and releases hydrochloric acid during the course of reaction. Therefore, only a small amount of hydrochloric acid is required to initiate the reaction.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




