
In a tetragonal crystal,
A)$\alpha =\beta ={{90}^{0}}\ne \gamma ,a=b=c$
B)$\alpha =\beta =\gamma ={{90}^{0}},a=b\ne c$
C) $\alpha =\beta =\gamma ={{90}^{0}},a\ne b\ne c$
D) $\alpha =\beta ={{90}^{0}},\gamma ={{120}^{0}},a=b\ne c$
Answer
566.7k+ views
Hint: The answer to this question is based on the concept of inorganic chemistry and the correct answer can be approached when the tetragonal structure is drawn that is by knowing the crystal lattice structure.
Complete Solution :
Now, let us see what a crystal structure is and how the structures can be assigned.
The crystal structure is a description of the ordered arrangements of atoms or ions or even molecules in the crystalline material.
- Unit cell gives the information of length of cell edges given by a, b, c and the angles between them denoted by $\alpha ,\beta ,\gamma $
Now, the tetragonal crystal system which is the stretched form of the cubic lattice system and the lattices are given by the Bravais lattice which are familiar to us. The tetragonal structure is as shown below”
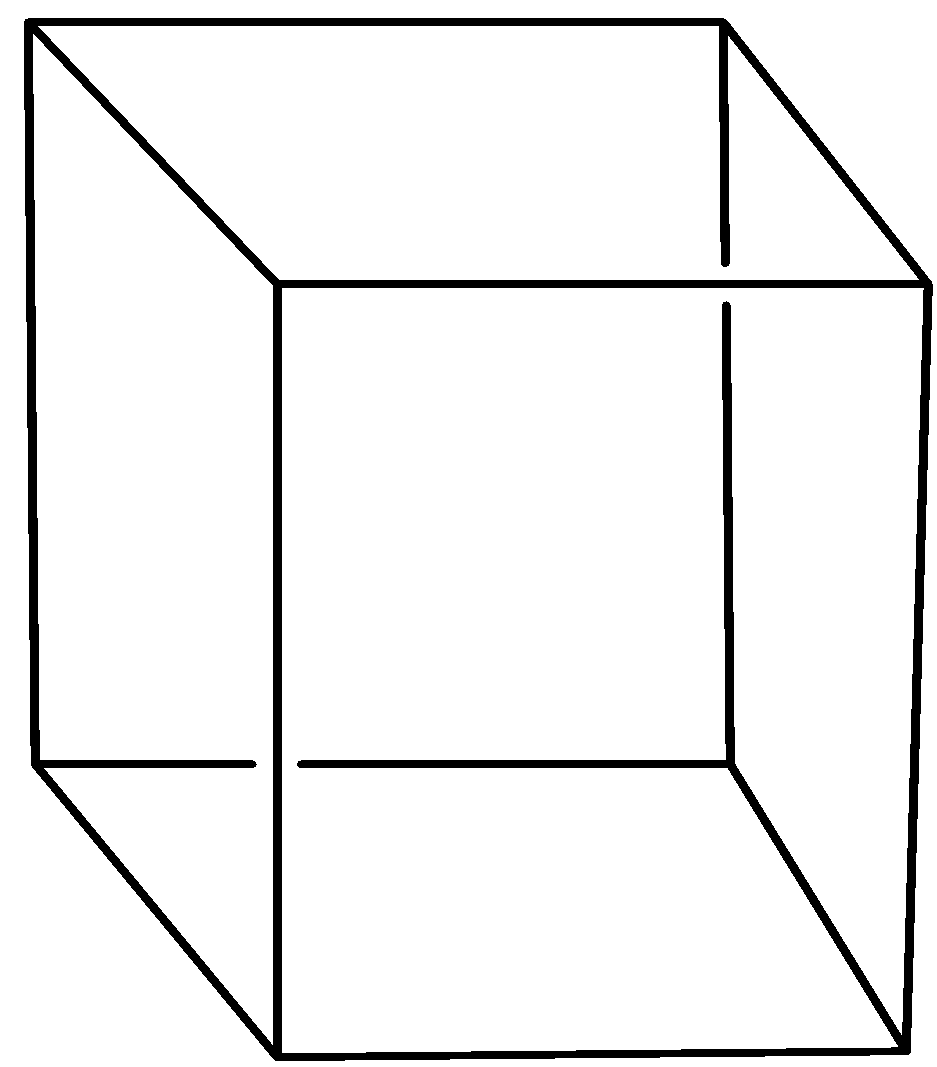
From this above image we can easily find that all the three bond angles are equal that is the bond angles $\alpha ,\beta ,\gamma $ are all ${{90}^{0}}$ and only lower two sides are equal that is a and b which are lying on same plane and the third side is on the other plane with $a=b\ne c$.
Therefore the correct answer is option $\alpha =\beta =\gamma ={{90}^{0}},a=b\ne c$
So, the correct answer is “Option B”.
Note: Note that unit cell is the smallest group of particles in the material that constitutes the repeating pattern and these crystal structures are described in terms of geometry of arrangement of the small particles in the unit cells and geometry of the unit cell is defined as parallelepiped.
Complete Solution :
Now, let us see what a crystal structure is and how the structures can be assigned.
The crystal structure is a description of the ordered arrangements of atoms or ions or even molecules in the crystalline material.
- Unit cell gives the information of length of cell edges given by a, b, c and the angles between them denoted by $\alpha ,\beta ,\gamma $
Now, the tetragonal crystal system which is the stretched form of the cubic lattice system and the lattices are given by the Bravais lattice which are familiar to us. The tetragonal structure is as shown below”

From this above image we can easily find that all the three bond angles are equal that is the bond angles $\alpha ,\beta ,\gamma $ are all ${{90}^{0}}$ and only lower two sides are equal that is a and b which are lying on same plane and the third side is on the other plane with $a=b\ne c$.
Therefore the correct answer is option $\alpha =\beta =\gamma ={{90}^{0}},a=b\ne c$
So, the correct answer is “Option B”.
Note: Note that unit cell is the smallest group of particles in the material that constitutes the repeating pattern and these crystal structures are described in terms of geometry of arrangement of the small particles in the unit cells and geometry of the unit cell is defined as parallelepiped.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




