
In a cyclotrimetaphosphoric acid molecule, how many single and double bonds are present?
(A) 3 double bonds, 9 single bonds
(B) 6 double bonds, 6 single bonds
(C) 3 double bonds, 12 single bonds
(D) Zero double bonds, 12 single bonds
Answer
587.7k+ views
Hint: The acids containing the element oxygen are oxoacids. These oxoacids contain at least one other element and at least one hydrogen atom bonded to an oxygen atom. Like phosphorus, sulfur, and fluorine will form this type of oxoacids. Sometimes these oxoacids are also called oxyacids.
Complete step by step answer:
Phosphorus forms many oxoacids are ${{H}_{3}}P{{O}_{2}},{{H}_{3}}P{{O}_{4}},{{H}_{3}}P{{O}_{4}}$ , etc. In oxoacids of phosphorus, P is tetrahedrally surrounded by other atoms. All of these are known to form at least one P-H bond and an O-H bond.
P-P bonds or P-H bonds are also found including P=O bonds in poly oxoacids of phosphorus. The oxidation number of P is less than +5 in oxoacids of phosphorus.
Cyclotrimetaphosphoric acid molecule is a type of metaphosphoric acid the molecular formula is ${{(HP{{O}_{3}})}_{3}}$ and the structure is:
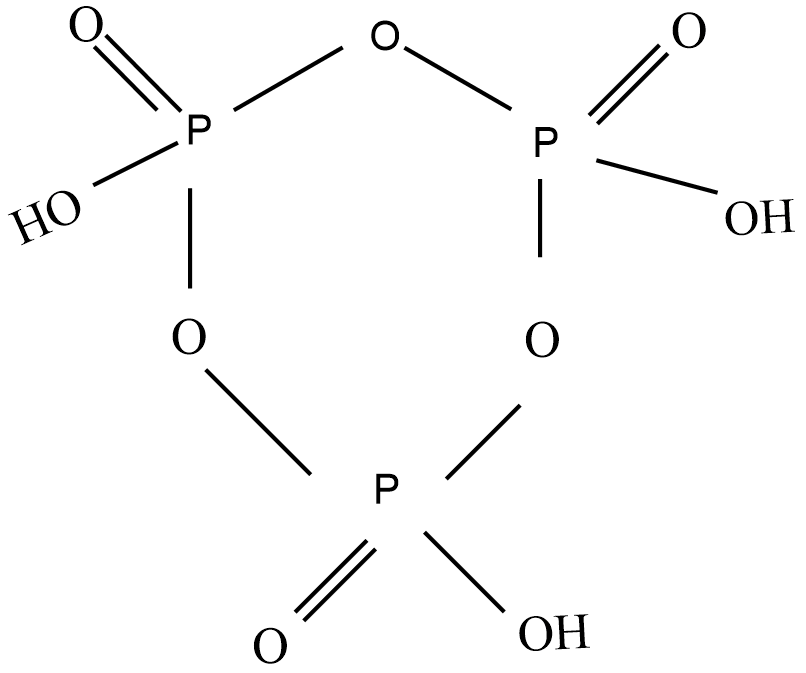
From the above structure of Cyclotrimetaphosphoric acid molecule, there are 3 P=O bonds, 3 P-OH bonds, and 3 O-P-O bonds. Hence, a total of 3 double bonds and12 single bonds.So, Cyclotrimetaphosphoric acid molecule has 3 double bonds and 12 single bonds.
So, the correct answer is “Option C”.
Note: The oxoacids of phosphorus which contain P-H bonds have strong reducing agents. Out of all oxoacids of phosphorus, hypophosphorous is a strong reducing agent, which contains two P-H bonds and reduces.
Complete step by step answer:
Phosphorus forms many oxoacids are ${{H}_{3}}P{{O}_{2}},{{H}_{3}}P{{O}_{4}},{{H}_{3}}P{{O}_{4}}$ , etc. In oxoacids of phosphorus, P is tetrahedrally surrounded by other atoms. All of these are known to form at least one P-H bond and an O-H bond.
P-P bonds or P-H bonds are also found including P=O bonds in poly oxoacids of phosphorus. The oxidation number of P is less than +5 in oxoacids of phosphorus.
| Name | Formula | The oxidation state of Phosphorous | Characteristics of bonds and their number |
| hypo phosphorous | ${{H}_{3}}P{{O}_{2}}$ | +1 | One P-OH,Two P-H,One P=O |
| Ortho phosphorous | ${{H}_{3}}P{{O}_{3}}$ | +3 | two P-OH,one P-H,One P=O |
| Pyro phosphoric acid | ${{H}_{3}}P{{O}_{4}}$ | +5 | Three P-H,One P=O |
| Metaphosphoric acid | ${{(HP{{O}_{3}})}_{n}}$ | +5 | Three p-OH,Three P=O,Three P-O-P |
Cyclotrimetaphosphoric acid molecule is a type of metaphosphoric acid the molecular formula is ${{(HP{{O}_{3}})}_{3}}$ and the structure is:

From the above structure of Cyclotrimetaphosphoric acid molecule, there are 3 P=O bonds, 3 P-OH bonds, and 3 O-P-O bonds. Hence, a total of 3 double bonds and12 single bonds.So, Cyclotrimetaphosphoric acid molecule has 3 double bonds and 12 single bonds.
So, the correct answer is “Option C”.
Note: The oxoacids of phosphorus which contain P-H bonds have strong reducing agents. Out of all oxoacids of phosphorus, hypophosphorous is a strong reducing agent, which contains two P-H bonds and reduces.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




