
What important information is furnished about the nucleus of an atom by the alpha particle scattering experiment of Rutherford?
Answer
515.4k+ views
Hint: The Geiger–Marsden tests (also known as the Rutherford gold foil experiment) were a set of ground breaking tests that demonstrated that every atom has a nucleus that contains all of its positive charge and most of its mass. They came at this conclusion after seeing how an alpha particle beam scatters when it collides with a thin metal foil. The tests were carried out at the University of Manchester's Physical Laboratories between 1908 and 1913 by Hans Geiger and Ernest Marsden under the supervision of Ernest Rutherford.
Complete answer:
Geiger and Marsden, at Rutherford's request, conducted a series of experiments in which they directed an alpha particle beam against a thin metal foil and observed the scattering pattern using a fluorescence screen. They noticed alpha particles bouncing in all directions off the metal foil, some right back to the source. According to Thomson's hypothesis, all of the alpha particles should have gone straight through. Those particles had obviously faced an electric force substantially stronger than Thomson's model predicted. In addition, only a small percentage of alpha particles were deflected by greater than 90 degrees.
The majority of them sailed straight through the foil with minimal deflection. To explain this strange finding, Rutherford hypothesised that the atom's positive charge was concentrated in a small nucleus at its core, leaving most of the atom's volume unoccupied.
When alpha particles are permitted to travel through an atom, they are totally deflected back in the region where the nucleus is present.
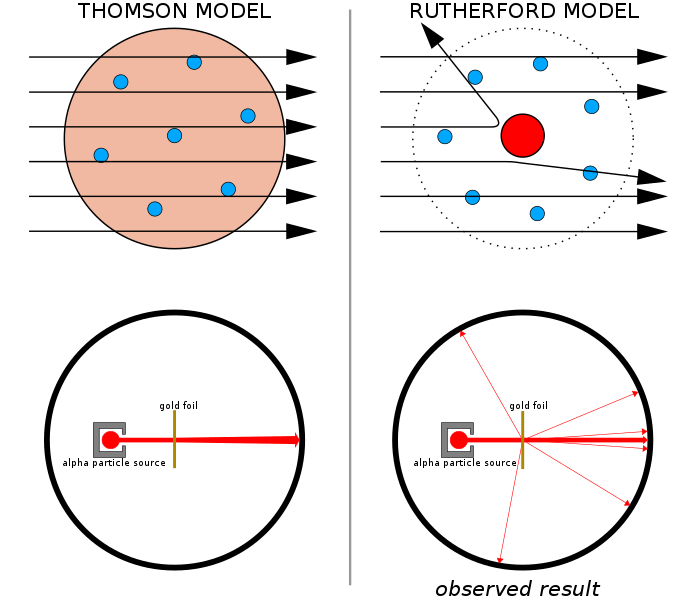
This demonstrates that the nucleus contains the whole mass of the atom and is made up of positively charged particles.
The postulates of Rutherford's alpha-particle scattering experiment are as follows:
(i). An atom's nucleus is positively charged.
(ii) The nucleus of an atom is very dense and rigid.
iii) The nucleus of an atom is extremely tiny in comparison to the overall size of the atom.
Note:
Rutherford thought that the atom's core charge was positive in his 1911 publication, although a negative charge would have matched his scattering model just as well. Based on the results of studies studying the scattering of alpha particles in various gases, Rutherford concluded in a 1913 publication that the "nucleus" (as he now termed it) was certainly positively charged.
Complete answer:
Geiger and Marsden, at Rutherford's request, conducted a series of experiments in which they directed an alpha particle beam against a thin metal foil and observed the scattering pattern using a fluorescence screen. They noticed alpha particles bouncing in all directions off the metal foil, some right back to the source. According to Thomson's hypothesis, all of the alpha particles should have gone straight through. Those particles had obviously faced an electric force substantially stronger than Thomson's model predicted. In addition, only a small percentage of alpha particles were deflected by greater than 90 degrees.
The majority of them sailed straight through the foil with minimal deflection. To explain this strange finding, Rutherford hypothesised that the atom's positive charge was concentrated in a small nucleus at its core, leaving most of the atom's volume unoccupied.
When alpha particles are permitted to travel through an atom, they are totally deflected back in the region where the nucleus is present.

This demonstrates that the nucleus contains the whole mass of the atom and is made up of positively charged particles.
The postulates of Rutherford's alpha-particle scattering experiment are as follows:
(i). An atom's nucleus is positively charged.
(ii) The nucleus of an atom is very dense and rigid.
iii) The nucleus of an atom is extremely tiny in comparison to the overall size of the atom.
Note:
Rutherford thought that the atom's core charge was positive in his 1911 publication, although a negative charge would have matched his scattering model just as well. Based on the results of studies studying the scattering of alpha particles in various gases, Rutherford concluded in a 1913 publication that the "nucleus" (as he now termed it) was certainly positively charged.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




