
Illustrate a glycosidic, peptide, and phosphodiester bond.
Answer
578.4k+ views
Hint: All these bonds are present between organic compounds and they are necessary for forming all the basic components which support life for all organisms, from single-celled bacteria to multicellular human beings.
Complete answer:
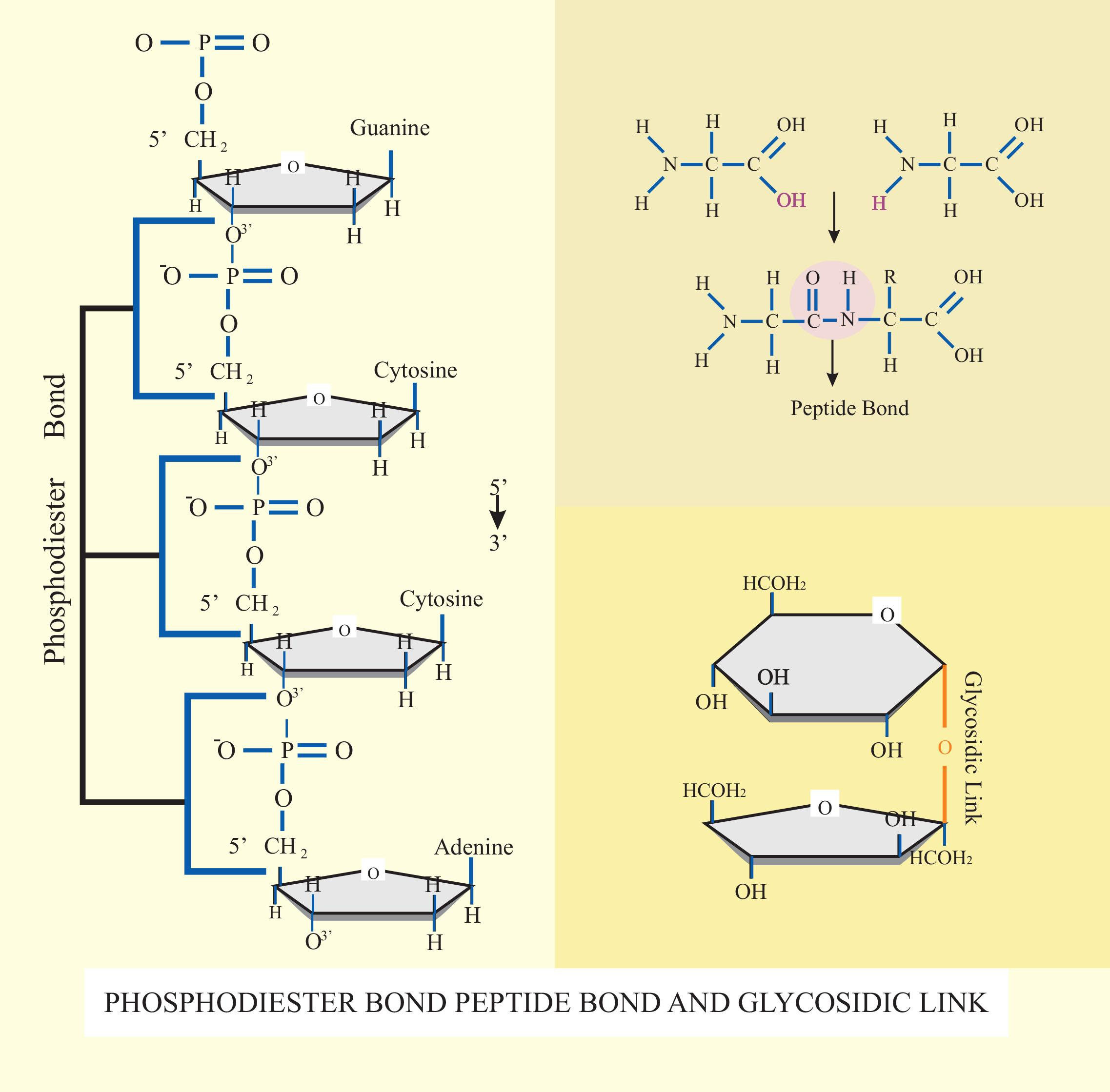
Glycosidic bond: The bond present between the hydroxyl group of certain compounds and groups of saccharide is known as a glycosidic bond.
-These bonds are present in certain inorganic compounds such as the sulfuric acid and any substance which has a glycosidic bond is known as a glycoside.
Peptide bond: Peptide bond is also known as the eupeptide bond which bounds among two peptides or proteins.
-A dipeptide formed through a peptide bond and two amino acids are a type of condensation reaction.
-In organisms, the organisms provide ATP for the formation of a peptide bond which demands energy.
-Phosphodiester bond: Phosphodiester bond is universal to all life forms present on earth because of the backbone of both DNA and RNA.
-By alkaline hydrolysis, the phosphodiester bond inside two ribonucleotides can be broken but deoxyribonucleotides have a better stable linkage in these conditions. All three bonds are found in all the organic compounds present in human beings.
Note: -Phosphodiesterase is an enzyme that catalyzes the hydrolysis of the phosphodiester bond which plays an important role in repairing DNA sequences.
-The nitrogen-carbon linkage present in DNA is glycosidic. Damages or aberrations occurring in the generic content can cause severe genetic related diseases and mutations.
-While the replication of DNA occurs there will be damage to the genetic content which is fixed by phosphodiesterase enzyme.
Complete answer:
Glycosidic bond: The bond present between the hydroxyl group of certain compounds and groups of saccharide is known as a glycosidic bond.
-These bonds are present in certain inorganic compounds such as the sulfuric acid and any substance which has a glycosidic bond is known as a glycoside.
Peptide bond: Peptide bond is also known as the eupeptide bond which bounds among two peptides or proteins.
-A dipeptide formed through a peptide bond and two amino acids are a type of condensation reaction.
-In organisms, the organisms provide ATP for the formation of a peptide bond which demands energy.
-Phosphodiester bond: Phosphodiester bond is universal to all life forms present on earth because of the backbone of both DNA and RNA.
-By alkaline hydrolysis, the phosphodiester bond inside two ribonucleotides can be broken but deoxyribonucleotides have a better stable linkage in these conditions. All three bonds are found in all the organic compounds present in human beings.

Note: -Phosphodiesterase is an enzyme that catalyzes the hydrolysis of the phosphodiester bond which plays an important role in repairing DNA sequences.
-The nitrogen-carbon linkage present in DNA is glycosidic. Damages or aberrations occurring in the generic content can cause severe genetic related diseases and mutations.
-While the replication of DNA occurs there will be damage to the genetic content which is fixed by phosphodiesterase enzyme.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




