
If x is the length of body diagonal, then the distance between two nearest cations in rock salt structure is:
A.$\dfrac{X}{{\sqrt 6 }}$
B.$\dfrac{X}{{\sqrt 5 }}$
C.$\dfrac{X}{{\sqrt 3 }}$
D.$\dfrac{X}{{\sqrt 2 }}$
Answer
581.1k+ views
Hint: The rock salt structure has the edges of all the same measurement that means its length, breadth and height are the same. The diagonal is the one which cuts through the middle of the cube but this is not the main diagonal. The value of the main diagonal can be calculated by multiplying the value of length of one side by the $\sqrt 3 $.
Complete step by step answer:
The NaCl crystal structure is face centered cubic.
The total number of ions available in one unit cell of NaCl crystal lattice is eight which includes four sodium ions $N{a^ + }$ and four chloride ions $C{l^ - }$.
In the crystalline structure eight $C{l^ - }$ ions are present at the eight corners of the face centered cubic unit cell and six $C{l^ - }$ ions are present at six face centers.
The four $N{a^ + }$ ion is available in the edge center and body center.
The lattice structure of NaCl is shown below.
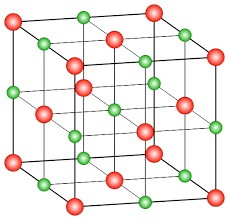
Assume that if $a$ is the edge length of the NaCl cube, then the body diagonal is given as shown below.
$d = \sqrt {3a} ......(i)$
It is given $d = x$
Substitute the value of d in equation (i).
$ \Rightarrow x = \sqrt {3a} ......(ii)$
As, the atoms present at the face center and the corner of the cube is adjacent to each other, therefore the distance between the two closest cation is given by
$ = \dfrac{{\sqrt {2a} }}{2}......(iii)$
Let us assume $\dfrac{a}{2}$ as ${d'}$.
${d'}$ is the distance between the two cations.
Equate equation (ii) and (iii), we get
$ \Rightarrow \dfrac{x}{{\sqrt 3 }} = \sqrt {2{d'}} $
$ \Rightarrow {d'} = \dfrac{x}{{\sqrt 6 }}$
Thus, the distance between two nearest cations in rock salt structure is $\dfrac{x}{{\sqrt 6 }}$
Therefore, the correct option is A.
Note:
Many compounds like AgCl, AgBr, KCl, RbCl and other alkaline earth metals oxides and sulphides resemble the structure of sodium chloride. The compound beryllium sulphide is an exception for this property.
Complete step by step answer:
The NaCl crystal structure is face centered cubic.
The total number of ions available in one unit cell of NaCl crystal lattice is eight which includes four sodium ions $N{a^ + }$ and four chloride ions $C{l^ - }$.
In the crystalline structure eight $C{l^ - }$ ions are present at the eight corners of the face centered cubic unit cell and six $C{l^ - }$ ions are present at six face centers.
The four $N{a^ + }$ ion is available in the edge center and body center.
The lattice structure of NaCl is shown below.

Assume that if $a$ is the edge length of the NaCl cube, then the body diagonal is given as shown below.
$d = \sqrt {3a} ......(i)$
It is given $d = x$
Substitute the value of d in equation (i).
$ \Rightarrow x = \sqrt {3a} ......(ii)$
As, the atoms present at the face center and the corner of the cube is adjacent to each other, therefore the distance between the two closest cation is given by
$ = \dfrac{{\sqrt {2a} }}{2}......(iii)$
Let us assume $\dfrac{a}{2}$ as ${d'}$.
${d'}$ is the distance between the two cations.
Equate equation (ii) and (iii), we get
$ \Rightarrow \dfrac{x}{{\sqrt 3 }} = \sqrt {2{d'}} $
$ \Rightarrow {d'} = \dfrac{x}{{\sqrt 6 }}$
Thus, the distance between two nearest cations in rock salt structure is $\dfrac{x}{{\sqrt 6 }}$
Therefore, the correct option is A.
Note:
Many compounds like AgCl, AgBr, KCl, RbCl and other alkaline earth metals oxides and sulphides resemble the structure of sodium chloride. The compound beryllium sulphide is an exception for this property.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




