
If optical rotation produced by the compound [A] is $+{{65}^{\circ }}$, then produced by the compound [B] is?
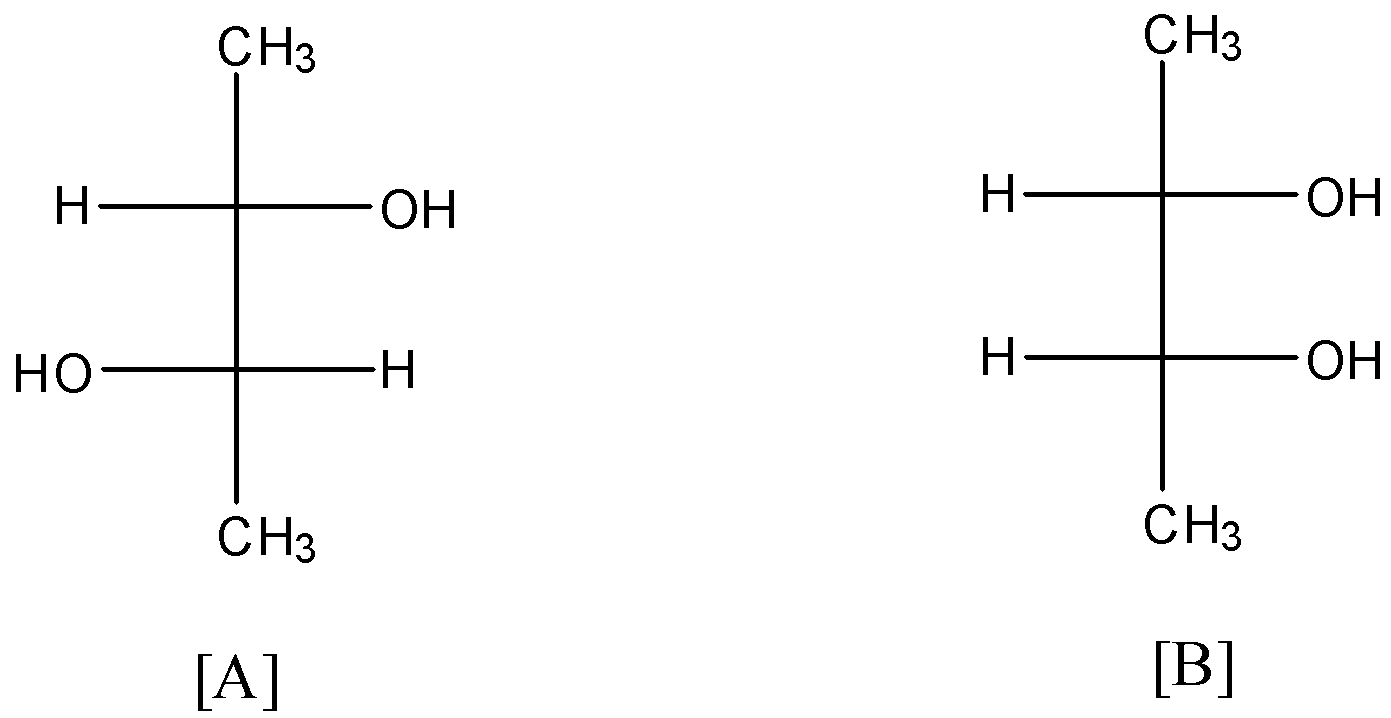
(a)- $+{{65}^{\circ }}$
(b)- $-{{65}^{\circ }}$
(c)- Zero
(d)- Unpredictable

Answer
588.6k+ views
Hint: The optical rotation of the compound is caused by the presence of chiral carbon. The compound will show optical rotation only if it does not have any symmetry. If the compound has asymmetry then it will be optically inactive and will have zero rotation.
Complete step by step answer:
Certain substances rotate the plane of polarized light when plane-polarized light is passed through their solution.
So, we can say that the substance which can rotate the plane of polarized light are called optically active substances.
A polarimeter is an instrument which is used to measure the angle of the plane of polarized light that is rotated.
A molecule which contains an asymmetric carbon lacks all elements of symmetry and hence is called an asymmetric molecule and is it this asymmetry of the molecule which is responsible for optical activity in such an organic compound.
But in some molecules are optically inactive even there is the presence of an asymmetric carbon atom. This inactivity of the compound is due to the presence of overall symmetry in the molecule.
In [B]:
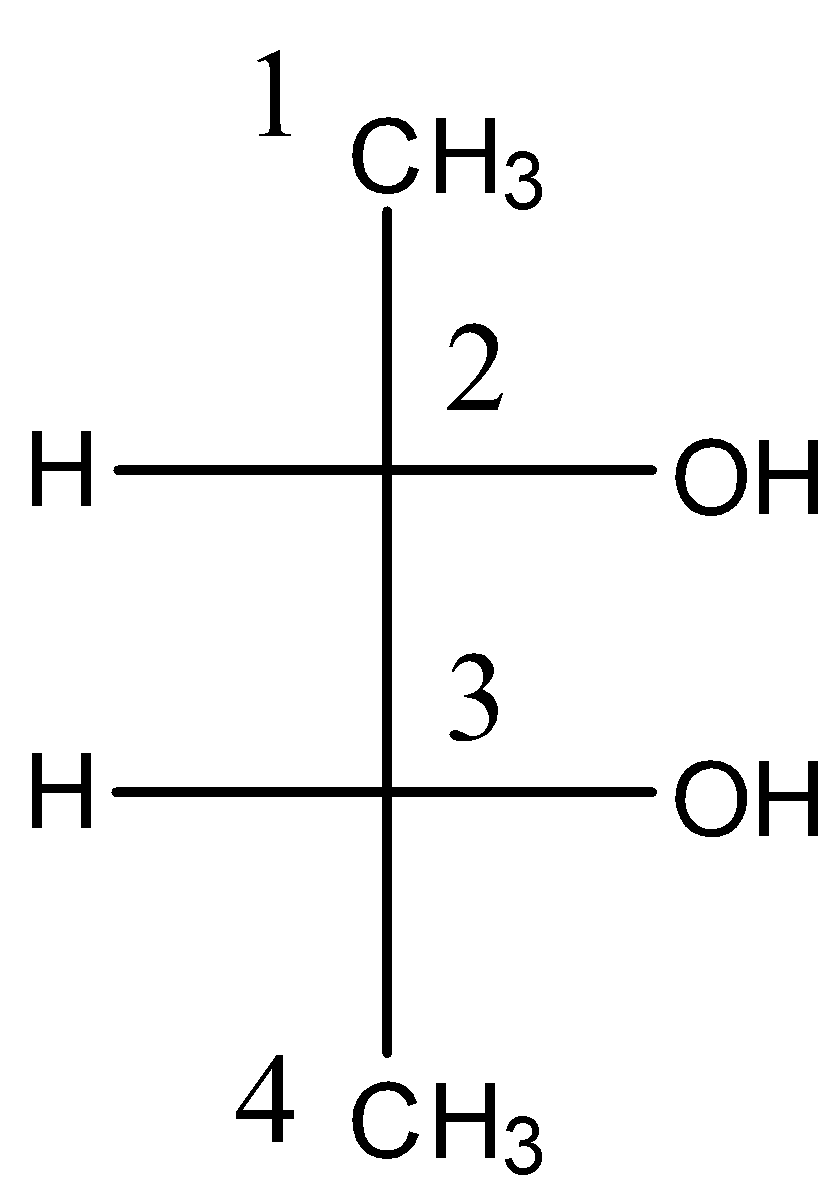
There are 2 chiral or asymmetric carbons present in positions 2 and 3. And this compound also has a plane of symmetry as shown below:
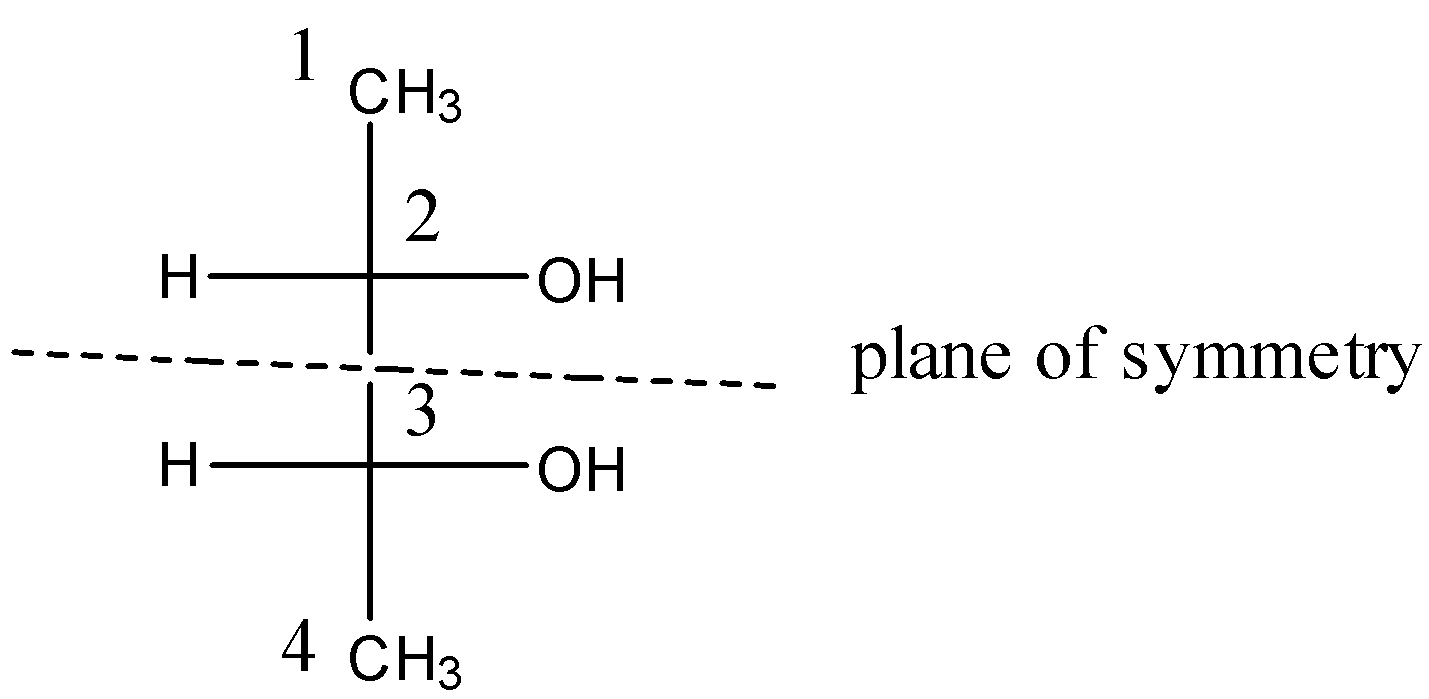
So, due to this optical inactivity, the compound [B] will have zero rotation.
So, the correct answer is an option (c)- Zero.
Note: We can say that compounds that do not show optical activity despite the presence of chiral carbon atoms are called Meso compounds. So, the compound [B] is a Meso compound.
Complete step by step answer:
Certain substances rotate the plane of polarized light when plane-polarized light is passed through their solution.
So, we can say that the substance which can rotate the plane of polarized light are called optically active substances.
A polarimeter is an instrument which is used to measure the angle of the plane of polarized light that is rotated.
A molecule which contains an asymmetric carbon lacks all elements of symmetry and hence is called an asymmetric molecule and is it this asymmetry of the molecule which is responsible for optical activity in such an organic compound.
But in some molecules are optically inactive even there is the presence of an asymmetric carbon atom. This inactivity of the compound is due to the presence of overall symmetry in the molecule.
In [B]:

There are 2 chiral or asymmetric carbons present in positions 2 and 3. And this compound also has a plane of symmetry as shown below:

So, due to this optical inactivity, the compound [B] will have zero rotation.
So, the correct answer is an option (c)- Zero.
Note: We can say that compounds that do not show optical activity despite the presence of chiral carbon atoms are called Meso compounds. So, the compound [B] is a Meso compound.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




