
If HCl is added over $ \left [ CH_2=C{(CH_3)}_2r \right ]$ then what is formed
A. $CH_2Cl=C{(CH_3)}_2$
B. $CH_3-C{(CH_3)}_2Cl$
C. $CH_2=C(CH_3)(CH_2)Cl$
D. None of these
Answer
361.8k+ views
Hint: Addition of alkenes follows markovnikov’s rule according to markovnikov’s rule the attacking nucleophile gets attached to the carbon that is containing less number of hydrogen. The reaction will proceed with the mechanism of the classical carbocation.
Complete Step by Step Answer:
As we know during the addition reaction the formation of carbocation takes place as the carbocation is formed the positive charge move to the carbon at which the most stable carbocation will be formed or we can say that the positive charge will stay on the carbon that will have more no of substituent attached that will have +I effect. So, the stability of primary, secondary, and tertiary carbocation will be-
${CH_3}^+
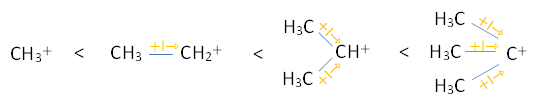
So here we can see that the most stable carbocation is tertiary carbocation because it has three methyl groups attached with it.
The following reaction will be:
$CH_2=C{(CH_3)}_2+H-Cl\longrightarrow CH_3-C{(CH_3)}_2Cl$
The mechanism of reaction will be:
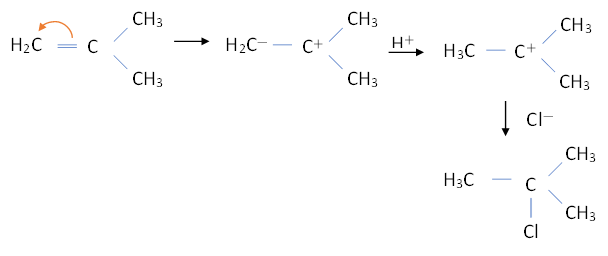
So, we can see that the product formed from the given reactants is 1-chloro,1-methylpropane. This reaction follows markovnikov’s rule for addition in the alkenes.
In addition to the reaction of alkene the pi(π) bond between the two carbons of alkene breaks and they form two sigma bonds with two separate chemical species. The geometry of the carbon is changed because it changes its hybridization from sp2 to sp3 so the shape changes from trigonal planar to tetrahedral.
Thus, Option (A) is correct
Note: Not all reactions follow markovnikov’s rule. In the second reaction 2- bromobutane contains a chiral carbon which means it will have stereoisomers. It will have two stereoisomers; they will be enantiomers that means mirror image of each other.
Complete Step by Step Answer:
As we know during the addition reaction the formation of carbocation takes place as the carbocation is formed the positive charge move to the carbon at which the most stable carbocation will be formed or we can say that the positive charge will stay on the carbon that will have more no of substituent attached that will have +I effect. So, the stability of primary, secondary, and tertiary carbocation will be-
${CH_3}^+

So here we can see that the most stable carbocation is tertiary carbocation because it has three methyl groups attached with it.
The following reaction will be:
$CH_2=C{(CH_3)}_2+H-Cl\longrightarrow CH_3-C{(CH_3)}_2Cl$
The mechanism of reaction will be:

So, we can see that the product formed from the given reactants is 1-chloro,1-methylpropane. This reaction follows markovnikov’s rule for addition in the alkenes.
In addition to the reaction of alkene the pi(π) bond between the two carbons of alkene breaks and they form two sigma bonds with two separate chemical species. The geometry of the carbon is changed because it changes its hybridization from sp2 to sp3 so the shape changes from trigonal planar to tetrahedral.
Thus, Option (A) is correct
Note: Not all reactions follow markovnikov’s rule. In the second reaction 2- bromobutane contains a chiral carbon which means it will have stereoisomers. It will have two stereoisomers; they will be enantiomers that means mirror image of each other.
Recently Updated Pages
Master Class 8 Social Science: Engaging Questions & Answers for Success

Master Class 8 English: Engaging Questions & Answers for Success

Class 8 Question and Answer - Your Ultimate Solutions Guide

Master Class 8 Maths: Engaging Questions & Answers for Success

Master Class 8 Science: Engaging Questions & Answers for Success

Master Class 7 English: Engaging Questions & Answers for Success

Trending doubts
What are the factors of 100 class 7 maths CBSE

Which are the Top 10 Largest Countries of the World?

What is BLO What is the full form of BLO class 8 social science CBSE

The value of 6 more than 7 is A 1 B 1 C 13 D 13 class 7 maths CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE




