
Identify Z in the following series ${C_2}{H_5}OH\xrightarrow{{PB{r_3}}}X\xrightarrow{{alc.KOH}}Y\xrightarrow{{dil.{H_2}S{O_4}}}Z$
$(A)C{H_2} = C{H_2}$
$(B)C{H_3}C{H_2}OH $
$(C)C{H_3} - C{H_2} - O - C{H_2} - C{H_3}$
(D)None
Answer
499.2k+ views
Hint: In these types of questions, we need to know step by step the product formed after the reaction in the presence of a catalyst. If we need to find the compound of $Z$, first we need to know the products formed at $X$ and $Y$.
Complete answer:
First we see the product formed after the reaction between ethanol and $PB{r_3}$. This reaction proceeds in two steps. In the first step the alcohol is converted into a good leaving group by forming a bond to $P$( $O - P$ bonds are very strong) and displacing $Br$ from $P$, this is essentially a nucleophilic substitution at phosphorus.
Now that the oxygen has converted to a good leaving group, a substitution reaction at carbon can occur. The bromide ion that is displaced from phosphorus attacks carbon via a backside attack, forming $C - Br$ and left with a new ethyl bromide. So, the $X$ is ${C_2}{H_5}Br$.
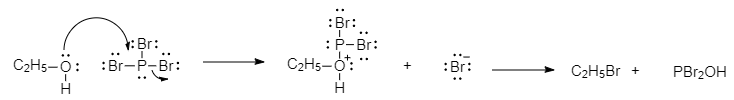
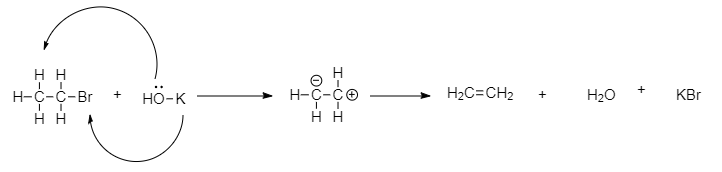
Now, ethyl bromide undergoes reaction in the presence of alcohol $KOH$, forming ethane. When ethyl bromide is boiled with $KOH$, the hydrogen atom transfers its electron pair to the adjacent carbon bond, and bromide is removed from the molecule. This forms a double bond between the alpha and beta carbon atoms and gives ethane as a product. So. The $Y$ is $C{H_2} = C{H_2}$.
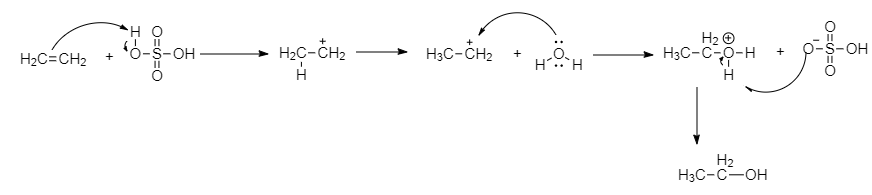
When ethane reacts with dilute sulphuric acid, it forms ethanol. All alkenes react with dilute sulphuric acid and give alcohol. This is a hydration reaction. A water molecule is added through double bonds and may give primary, secondary or tertiary alcohol. So the element $Z$, we get ethanol.
Therefore the correct answer is option B.
Note:
When haloalkane with $\beta $- hydrogen atoms are boiled with an alcoholic solution of potassium hydroxide, they undergo the elimination of hydrogen halide $(HX)$ resulting in the formation of alkenes.. The dilute sulphuric acid acts as a catalyst in hydrolysis of alkene.
Complete answer:
First we see the product formed after the reaction between ethanol and $PB{r_3}$. This reaction proceeds in two steps. In the first step the alcohol is converted into a good leaving group by forming a bond to $P$( $O - P$ bonds are very strong) and displacing $Br$ from $P$, this is essentially a nucleophilic substitution at phosphorus.
Now that the oxygen has converted to a good leaving group, a substitution reaction at carbon can occur. The bromide ion that is displaced from phosphorus attacks carbon via a backside attack, forming $C - Br$ and left with a new ethyl bromide. So, the $X$ is ${C_2}{H_5}Br$.
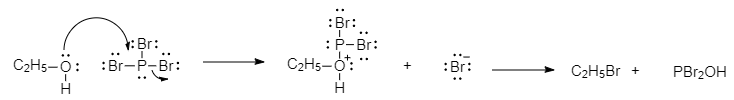
Now, ethyl bromide undergoes reaction in the presence of alcohol $KOH$, forming ethane. When ethyl bromide is boiled with $KOH$, the hydrogen atom transfers its electron pair to the adjacent carbon bond, and bromide is removed from the molecule. This forms a double bond between the alpha and beta carbon atoms and gives ethane as a product. So. The $Y$ is $C{H_2} = C{H_2}$.

When ethane reacts with dilute sulphuric acid, it forms ethanol. All alkenes react with dilute sulphuric acid and give alcohol. This is a hydration reaction. A water molecule is added through double bonds and may give primary, secondary or tertiary alcohol. So the element $Z$, we get ethanol.

Therefore the correct answer is option B.
Note:
When haloalkane with $\beta $- hydrogen atoms are boiled with an alcoholic solution of potassium hydroxide, they undergo the elimination of hydrogen halide $(HX)$ resulting in the formation of alkenes.. The dilute sulphuric acid acts as a catalyst in hydrolysis of alkene.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




