
Identify \[\;x,y\] and \[z\].



Answer
533.4k+ views
Hint: The nitro benzene chloride is the organic compound which a yellow crystalline solids. It is as important as the precursor to the other compounds because it consists of two functional groups. It has the tendency to dissolve in diethyl ether, benzene and hot ethanol. It does not get soluble in water.
Complete step by step answer:
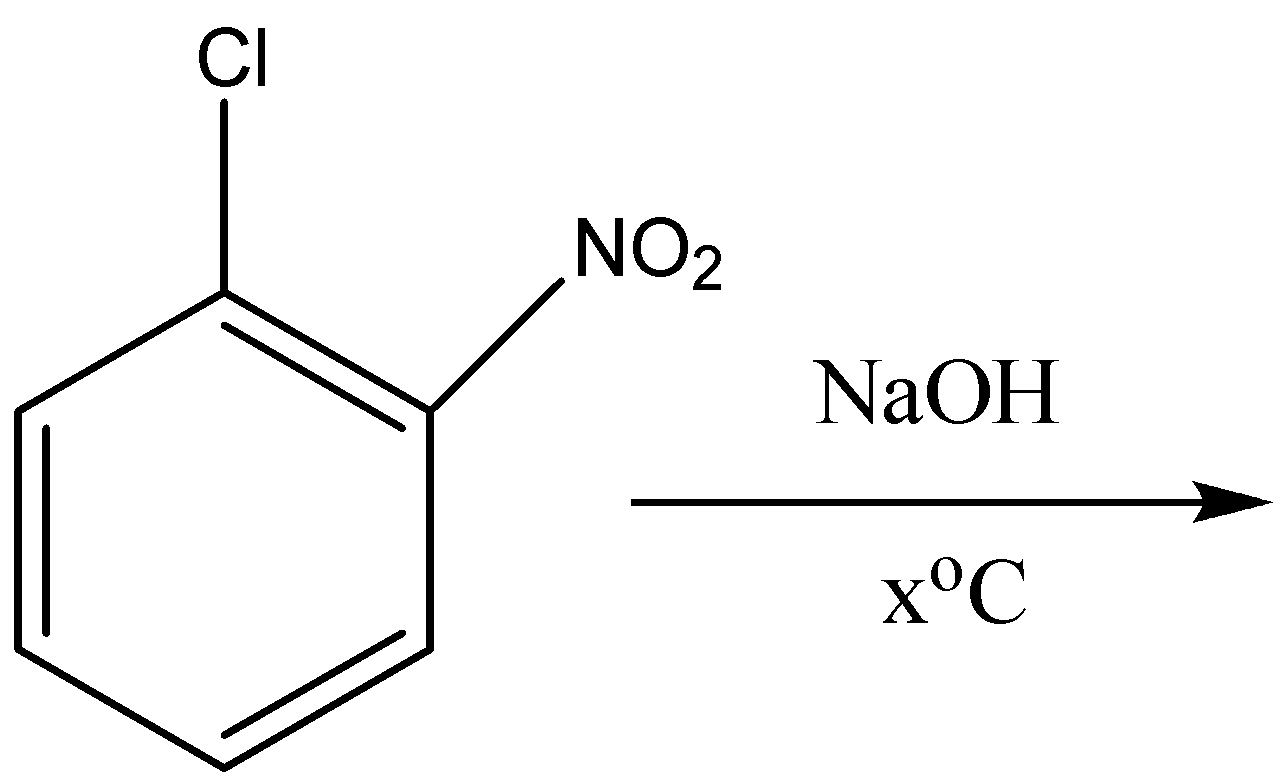
So when 2- nitrobenzene chloride reacts with sodium hydroxide at the temperature of \[443K\] or \[{170^o}C\] it forms 2- nitrophenol. So here the \[{x^o}C\] in the reaction or the equation is equal to \[{170^o}C\]. The equation for the reaction of 2- nitro benzene chloride is the following:
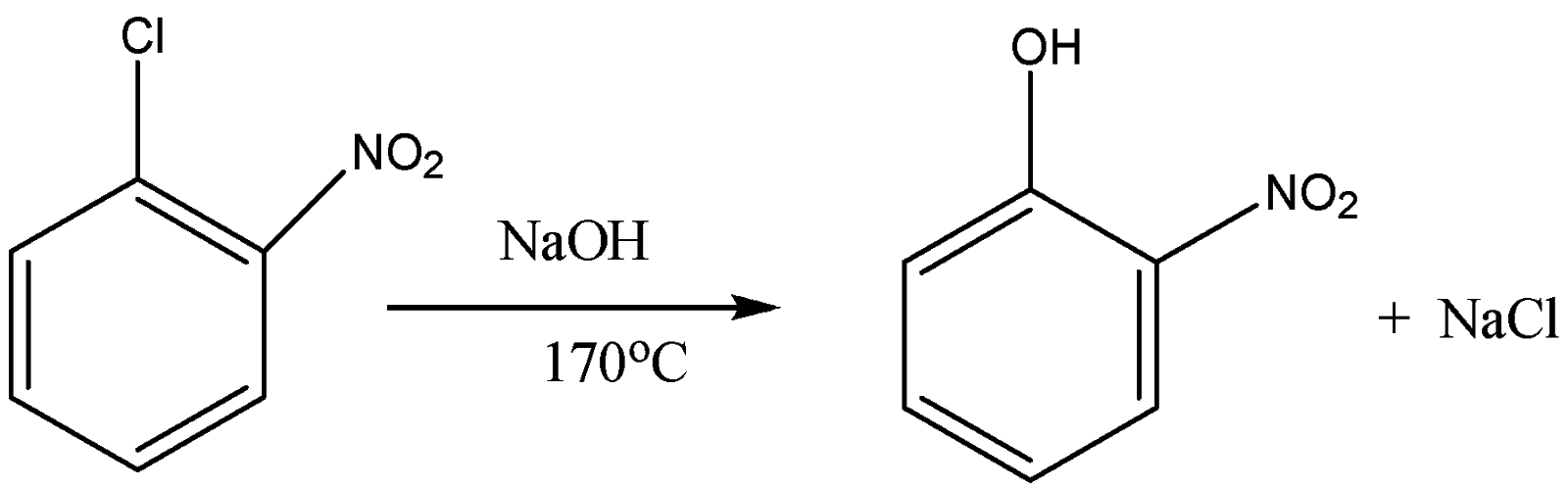
So the product formed is 2- nitrophenol and the answer for \[{x^o}C\] is \[{170^o}C\].
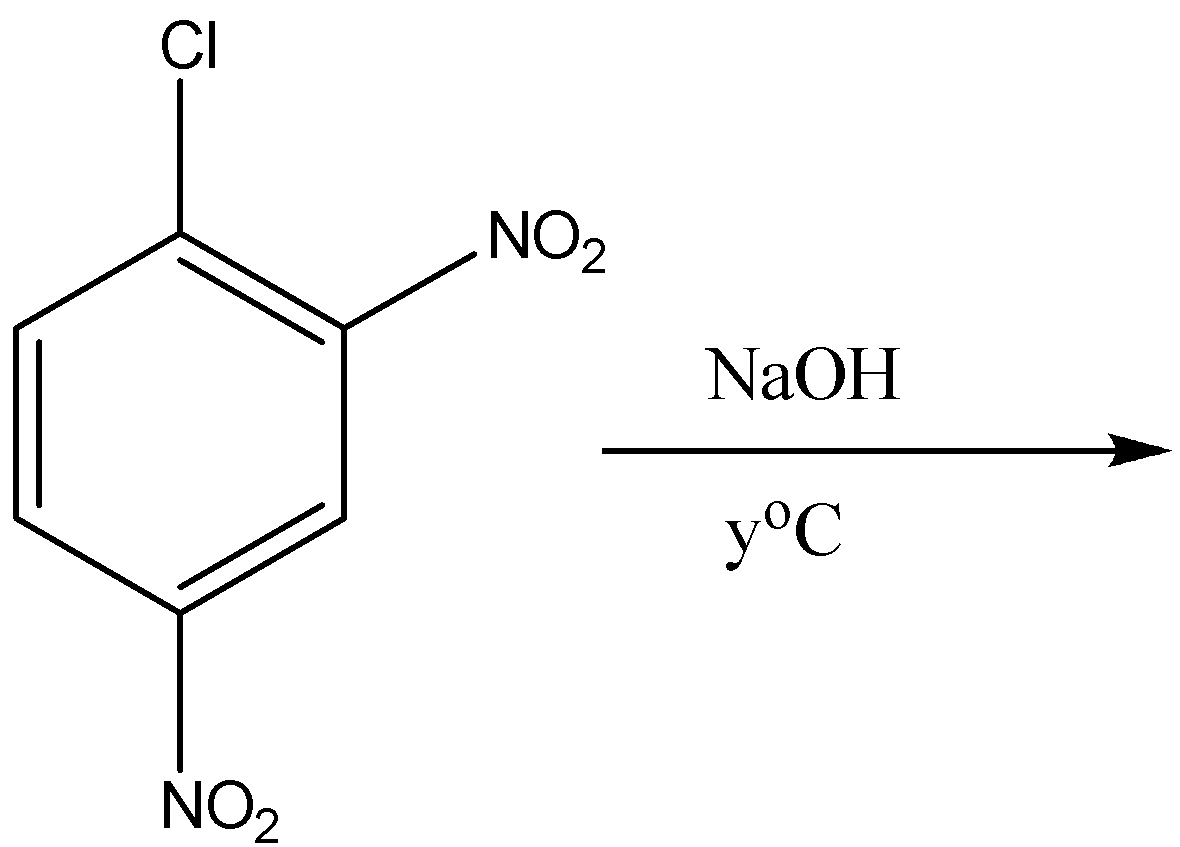
Now on coming to second part that is 2,4 dinitro benzene chloride, when it reacts with the sodium hydroxide at the temperature of \[{100^o}C\] or \[373K\] the formation of 2,4 dinitro phenol takes place. So the answer of \[{y^o}C\] in this reaction is \[{100^o}C\]. Now the equation for the 2,4-dinitro benzene chloride is the following:
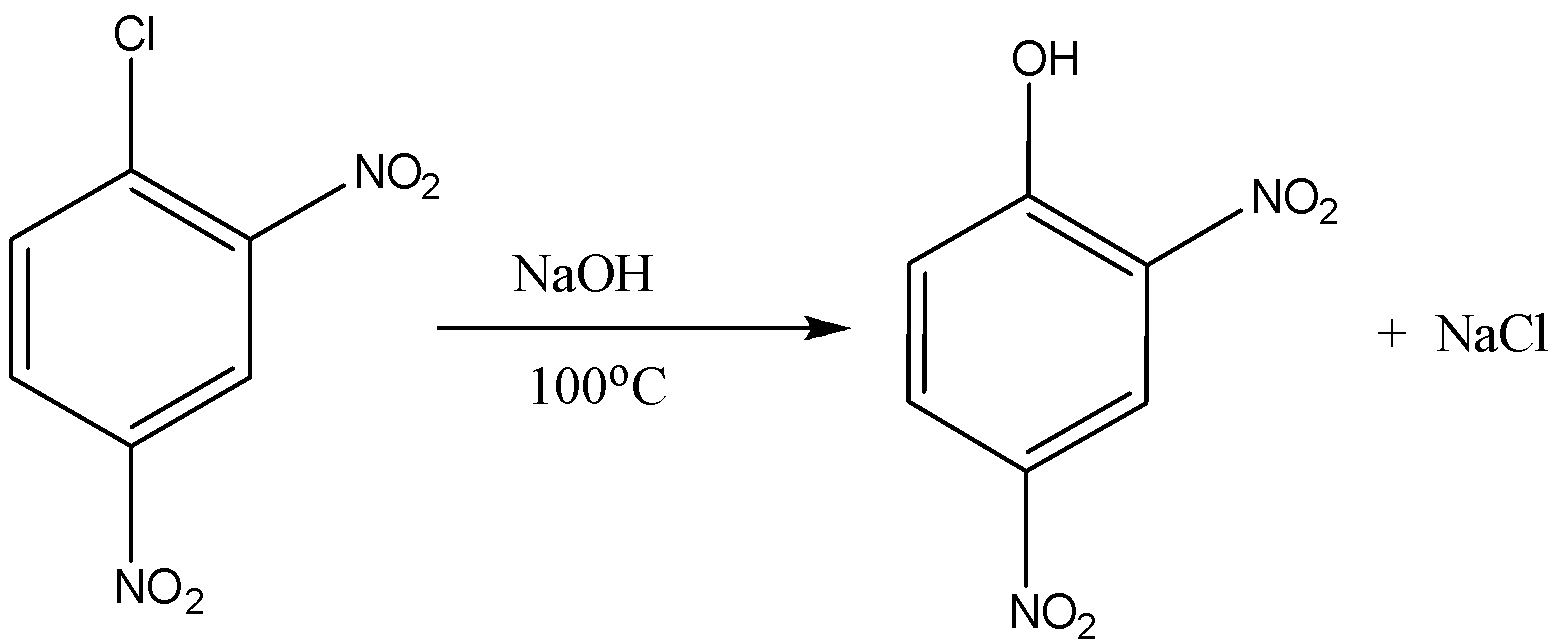
So the product formed is 2,4 dinitro phenol and the answer of \[{y^o}C\] is \[{100^o}C\].
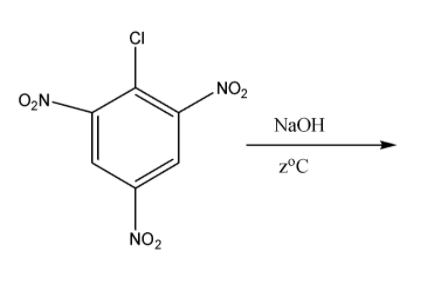
Now we come to the third part in which we have 2,4,6-trinitro benzene chloride. When it is reacted with sodium hydroxide with warm water at the temperature of \[333K\] or \[{60^o}C\] the formation of 2,4,6-tinitro phenol or picric acid takes place. The reaction for them is the following:
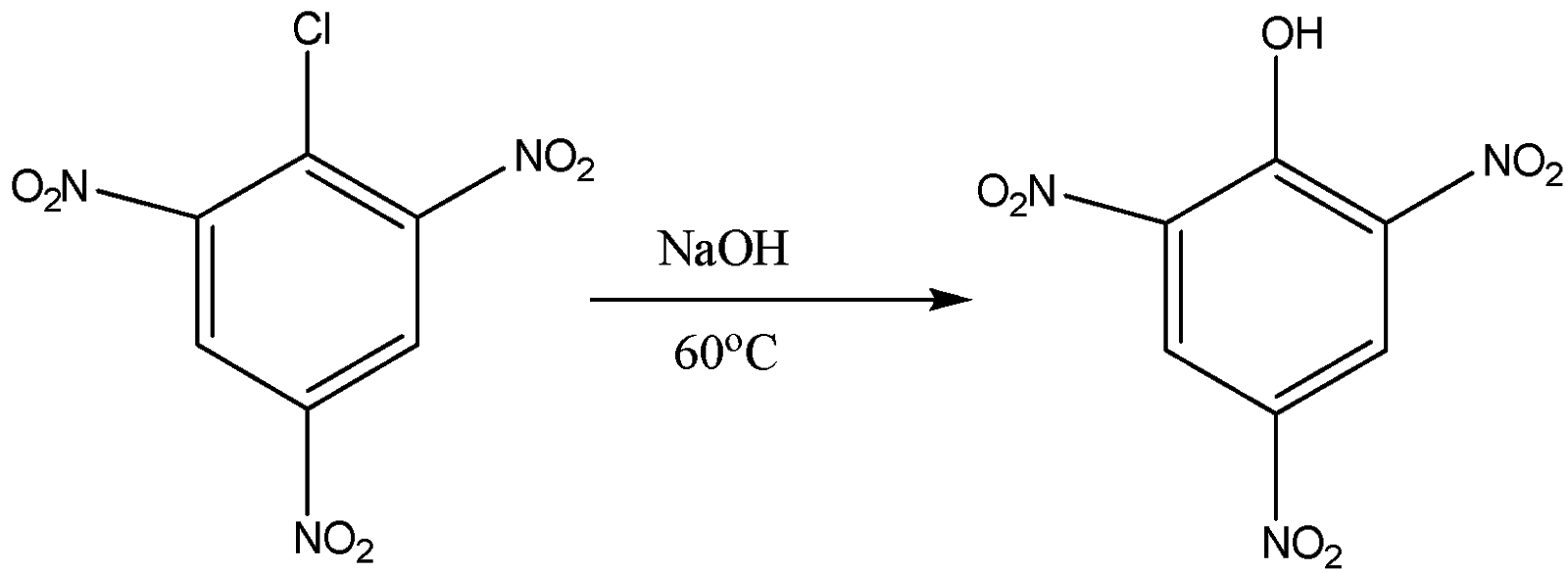
So the product formed is picric acid and the answer for \[{z^o}C\] is \[{60^o}C\].
Note: picric acid is toxic in nature and has the \[pH\] of 1.3. It is a strong acid and highly corrosive in nature as well as irritant to eyes too.it is pale yellow in colour which is a crystalline solid used in military explosives. It is used as a yellow dye and antiseptic. It is disposed of by incineration because it is dangerous.
Complete step by step answer:
So when 2- nitrobenzene chloride reacts with sodium hydroxide at the temperature of \[443K\] or \[{170^o}C\] it forms 2- nitrophenol. So here the \[{x^o}C\] in the reaction or the equation is equal to \[{170^o}C\]. The equation for the reaction of 2- nitro benzene chloride is the following:

So the product formed is 2- nitrophenol and the answer for \[{x^o}C\] is \[{170^o}C\].
Now on coming to second part that is 2,4 dinitro benzene chloride, when it reacts with the sodium hydroxide at the temperature of \[{100^o}C\] or \[373K\] the formation of 2,4 dinitro phenol takes place. So the answer of \[{y^o}C\] in this reaction is \[{100^o}C\]. Now the equation for the 2,4-dinitro benzene chloride is the following:

So the product formed is 2,4 dinitro phenol and the answer of \[{y^o}C\] is \[{100^o}C\].
Now we come to the third part in which we have 2,4,6-trinitro benzene chloride. When it is reacted with sodium hydroxide with warm water at the temperature of \[333K\] or \[{60^o}C\] the formation of 2,4,6-tinitro phenol or picric acid takes place. The reaction for them is the following:

So the product formed is picric acid and the answer for \[{z^o}C\] is \[{60^o}C\].
Note: picric acid is toxic in nature and has the \[pH\] of 1.3. It is a strong acid and highly corrosive in nature as well as irritant to eyes too.it is pale yellow in colour which is a crystalline solid used in military explosives. It is used as a yellow dye and antiseptic. It is disposed of by incineration because it is dangerous.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




